��Ŀ����
���ͷ���һ�ֻ�ѧ���ɼ�������С�մ��ۣ�̼����泥�������[KAl(SO4)2?12H2O]�е�����������ɡ�ijС��Ϊ̽����ͬƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣���������ʵ�顣
��������衿
��1������1����С�մ�ͳ������
����2����С�մ���������
����3����__________________________���
�����������̡�
Ϊ̽����Ʒ�Ƶķ��ͷ۵ijɷ֣�ijͬѧ�������ʵ�飬�õ���������
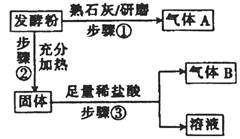
��2����ϲ���١��۷���������AΪ________���÷��ͷ۵ijɷ�Ϊ__________________��
��3��������ٺ͢ڲ������䣨����Ҳ��ͬ�����������������ϡ�����Ϊ�����Ȼ�����Һ���۲쵽�а�ɫ�������ɣ��ܷ�ȷ�����ͷ۵ijɷֲ�˵�����ɣ�________________�� ____________________________________________________________________��
��4����Ʒ�Ƶķ��ͷ۵Ļ�ѧ��ɿ���Ϊ����2����������ʵ����֤��
ʵ����������Ʒ��ѡ����ѡ�Լ���ϡ���ᡢ0.1 mol/LNaOH��Һ��д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
��������衿
��1������1����С�մ�ͳ������
����2����С�մ���������
����3����__________________________���
�����������̡�
Ϊ̽����Ʒ�Ƶķ��ͷ۵ijɷ֣�ijͬѧ�������ʵ�飬�õ���������

��2����ϲ���١��۷���������AΪ________���÷��ͷ۵ijɷ�Ϊ__________________��
��3��������ٺ͢ڲ������䣨����Ҳ��ͬ�����������������ϡ�����Ϊ�����Ȼ�����Һ���۲쵽�а�ɫ�������ɣ��ܷ�ȷ�����ͷ۵ijɷֲ�˵�����ɣ�________________�� ____________________________________________________________________��
��4����Ʒ�Ƶķ��ͷ۵Ļ�ѧ��ɿ���Ϊ����2����������ʵ����֤��
ʵ����������Ʒ��ѡ����ѡ�Լ���ϡ���ᡢ0.1 mol/LNaOH��Һ��д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ������Ʒ��������������� ����Һ�ֳ����ݣ��ֱ�װ��A��B�Թ��С� | |
| ����2��_____________________________ __________________________________ | ________________________֤����Na+���� �ͷ�����NaHCO3�� |
| ����3��_____________________________ ___________________________________ | ___________________________________ _______����ϲ���2�еĽ��ۣ�����2������ |
��16�֣���1�����ۺ�������2�֣�
��2����������NH3����2�֣� NaHCO3��NH4HCO3��2�֣�д����Ҳ���֣�
��3�����ܣ�1�֣�����Ϊ����NaHCO3�ֽ��Na2CO3��Na2CO3������������BaC12��Һ��Ӧ���ɰ�ɫ��������2�֣� ע����˼�ӽ�Ҳ�÷�
��4����7�֣�
������2����պȡA�е���Һ����պȡB��Һ�����ھƾ������������գ�1�֣�������ɫ�ܲ����۲죨1�֣� ��ɫ����ɫ��1�֣���֤�����ͷ��к���������1�֣�
ע������2�У����ýྻ�IJ�˿պȡ����Һ����1�֣����ھƾ��ƻ��������ա���1�֡�
ֻд��պȡ����Һ���ø��ֵ㲻�÷֣����ھƾ��ơ��ƾ���ƻ��������ա������֡�
����3�У�������ɫ��Ӧ����������ϴ�Ӳ�˿������NaOH��Һ���飬��0.lmol/L NaOH
��Һ����1�֣�����εμӡ���1�֡�
��2����������NH3����2�֣� NaHCO3��NH4HCO3��2�֣�д����Ҳ���֣�
��3�����ܣ�1�֣�����Ϊ����NaHCO3�ֽ��Na2CO3��Na2CO3������������BaC12��Һ��Ӧ���ɰ�ɫ��������2�֣� ע����˼�ӽ�Ҳ�÷�
��4����7�֣�
| ����2���ýྻ�IJ�˿պȡA�е���Һ���� �ƾ������������գ��۲���ɫ��2�֣� | ��ɫ�ʻ�ɫ��1�֣� |
| ����3��������1����B�Թ�����εμ� 0.1 mol/L NaOH��Һ��2�֣� | �����а�ɫ�������ɣ����Ȳ�����ɫ����������ܽ⣩��1�֣���֤�����ͷ�������������1�֣� |
������2����պȡA�е���Һ����պȡB��Һ�����ھƾ������������գ�1�֣�������ɫ�ܲ����۲죨1�֣� ��ɫ����ɫ��1�֣���֤�����ͷ��к���������1�֣�
ע������2�У����ýྻ�IJ�˿պȡ����Һ����1�֣����ھƾ��ƻ��������ա���1�֡�
ֻд��պȡ����Һ���ø��ֵ㲻�÷֣����ھƾ��ơ��ƾ���ƻ��������ա������֡�
����3�У�������ɫ��Ӧ����������ϴ�Ӳ�˿������NaOH��Һ���飬��0.lmol/L NaOH
��Һ����1�֣�����εμӡ���1�֡�
�����������1�����������֪����ͬƷ�Ƶķ��ͷۿ���С�մ�ͳ�����ɣ�Ҳ������С�մ��������ɣ��������ɳ��ۺ�������ɣ�������֪�ļ���1������2�ƶϼ���3Ϊ���ۺ���������2������NaHCO3��NH4HCO3��Al2(SO4)2?12H2O����Ҫ���ʿ�֪��NH4HCO3����Σ�����ʯ�һ����ĥ���Էų���������������������ʯ����ĥ�����ܷų����壬��AΪNH3�����ͷۼ�һ�����г��ۣ�NH4HCO3�����ּ��Ⱥ���ȫ��Ϊ�����ݳ���NaHCO3���������ȱ�ΪNa2CO3�����CO2��H2O��Na2CO3������������Ӧ���ɶ�����̼���塢NaCl��H2O����Al2(SO4)2?12H2O����ˮ�����Al(OH)3�������϶�������������ɴ��ƶ�BΪCO2���÷��ͷ�һ������С�մ����Լ�Ʒ�Ƶķ��ͷ۵���Ҫ�ɷ���NaHCO3��NH4HCO3����3��Ba(OH)2�����������η�Ӧ��������ɫ�����ᱵ������Ҳ����̼���η�Ӧ�����ɰ�ɫ��̼�ᱵ��������˲���ȷ�����ͷ��к�����������С�մ�4������ʵ�鷽���в���2�Ľ������ƿ�֪������2���ýྻ�IJ�˿պȡA�е���Һ���پƾ������������գ��������ɫ�ʻ�ɫ��֤����Na+�����ͷ�����NaHCO3�����ڼ���2�Ƿ��ͷ���С�մ��������ɣ�����3�Ľ�����֤�����ͷ���Al2(SO4)2?12H2O�����������ʼ��ṩ�Լ��������ƶϣ�����3�����ʵ�鷽��֤�����ͷ��к���Al3+�����Ӧ��B�Թ�����εμ�0.1mol/LNaOH��Һ���۲쵽��ɫ�������Ȳ�����ɫ����������ܽ⣬֤����Al3+�����ͷ�����������

��ϰ��ϵ�д�
 �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д�
�����Ŀ