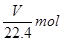��Ŀ����
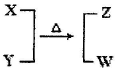
��12�֣�X��Y��Z��WΪ��������ͬ�ķ��ӻ����ӡ�X��5��ԭ�Ӻˡ�ͨ��״���£�WΪ��ɫҺ�塣����֮��ת����ϵ��ͼ��ʾ����ش�
��1����ҵ��ÿ��ȡ1molZҪ�ų�46.2 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2��ʵ������ȡZ�ķ�����ֹһ�֣�д������һ�ַ����Ļ�ѧ����ʽ�� ��
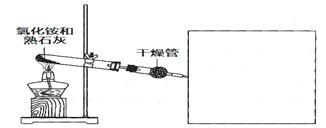
��3��ij��ѧС��ͬѧģ�ҵ������ȡHNO3�������ͼ��ʾװ�ã�����aΪһ���ɳ��������������Ƥ��
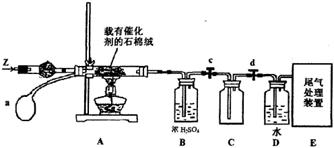
��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��B��ŨH2SO4�������� ��
��4��д��Dװ���з�Ӧ�Ļ�ѧ����ʽ ��
��5������ag HNO3��ϡ��Һ�У�����bg���۳�ַ�Ӧ������ȫ���ܽ⡣��֪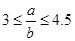 ����ԭ��HNO3������Ϊ g��
����ԭ��HNO3������Ϊ g��

��1����ҵ��ÿ��ȡ1molZҪ�ų�46.2 kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��2��ʵ������ȡZ�ķ�����ֹһ�֣�д������һ�ַ����Ļ�ѧ����ʽ�� ��
��3��ij��ѧС��ͬѧģ�ҵ������ȡHNO3�������ͼ��ʾװ�ã�����aΪһ���ɳ��������������Ƥ��

��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��B��ŨH2SO4�������� ��
��4��д��Dװ���з�Ӧ�Ļ�ѧ����ʽ ��
��5������ag HNO3��ϡ��Һ�У�����bg���۳�ַ�Ӧ������ȫ���ܽ⡣��֪
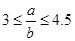 ����ԭ��HNO3������Ϊ g��
����ԭ��HNO3������Ϊ g����1��N2(g)+3H2(g) 2NH3(g) ��H="-92.4" kJ��mol
2NH3(g) ��H="-92.4" kJ��mol
��2��2NH4Cl+Ca(OH)2 2NH3��+2H2O+CaCl2��NH3��H2O
2NH3��+2H2O+CaCl2��NH3��H2O NH3��+H2O
NH3��+H2O
��3����4NH3+5O2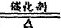 4NO+6H2O ������H2O��g����δ��Ӧ��NH3
4NO+6H2O ������H2O��g����δ��Ӧ��NH3
��4��3NO2+H2O=2HNO3+NO ��5��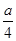
 2NH3(g) ��H="-92.4" kJ��mol
2NH3(g) ��H="-92.4" kJ��mol��2��2NH4Cl+Ca(OH)2
 2NH3��+2H2O+CaCl2��NH3��H2O
2NH3��+2H2O+CaCl2��NH3��H2O NH3��+H2O
NH3��+H2O��3����4NH3+5O2
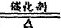 4NO+6H2O ������H2O��g����δ��Ӧ��NH3
4NO+6H2O ������H2O��g����δ��Ӧ��NH3��4��3NO2+H2O=2HNO3+NO ��5��
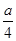
���������WΪ��ɫҺ�壬һ����H2O������10�����ӣ�X��5��ԭ�Ӻ�����10�����ӣ����ƶϳ���NH4������YΪOH����ZΪNH3��
��1����ҵ�Ϻϳɰ������������͵���ֱ�Ӻϳɣ���Ӧ����ʽΪN2(g)+3H2(g)
 2NH3(g) ��H="-92.4" kJ��mol
2NH3(g) ��H="-92.4" kJ��mol��2������ʵ�����Ʒ���һ���Ǽ�����κͼ�Ļ���
��3����4��������һϵ�з�Ӧ���Եõ����ᣬ����̿ɱ�ʾΪN2 ��NH3��NO��NO2��HNO3��
��5����������ʱ���˷�Ӧ�ķ���ʽΪ
4HNO3��Fe
 Fe(NO3)3��NO��2H2O
Fe(NO3)3��NO��2H2O4 1
b/14 b/56
��b/14��=(a/63) ��a/b=4.5
��������ʱ��ʣ������ܼ�����Fe(NO3)3��Ӧ���÷�Ӧ����ʽΪ
8HNO3��3Fe
 3Fe(NO3)2��2NO��4H2O
3Fe(NO3)2��2NO��4H2O8 3
b/56 b/21
�� b/21=(a/63) ��a/b=3
����Ϊ��a/b�����ڵ���3��С�ڵ���4.5��˵���÷�Ӧ���ڸղ����оٵ�����֮�䣬����Ӧ����ʽΪ
4Fe��12HNO3
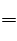 3Fe(NO3)2��Fe(NO3)3��3NO��6H2O
3Fe(NO3)2��Fe(NO3)3��3NO��6H2O12 3
a a/4
������ԭ�����������Ϊ(a/4)g��
����������ͨ���ƶϣ��жϳ�NԪ�ص�������ʣ���Ҫ�������Ϲ���NH3��HNO3���Ʒ��Ļ�ѧ����ʽ��ѧ�����Ը�����ѧ֪ʶ���н���ѶȲ���

��ϰ��ϵ�д�
�����Ŀ