��Ŀ����
9����һ�������½������·�Ӧ��aX��g��+bY��g��?cZ��g����ͼ1�Dz�ͬ�¶��·�Ӧ�ﵽƽ��ʱ����Ӧ�������Z�����������ѹǿ��ϵʾ��ͼ��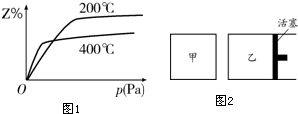
��1��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=$\frac{c{\;}^{c}��Z��}{c{\;}^{a}��X��•c{\;}^{b}��Y��}$�������¶ȵ����ߣ�Kֵ��С���������С�����䡱��������Ӧ����ʼŨ����ͬʱ��ƽ�ⳣ��KֵԽ����AB������ĸ����
A��X��ת����Խ�� B����Ӧ���е�Խ��ȫ
C���ﵽƽ��ʱX��Ũ��Խ�� D����ѧ��Ӧ����Խ��
��2����ͼ2��ʾ����ͬ�¶��£��ڼס����������и�Ͷ��1molX��2molY�������������ס����������ij�ʼ�����Ϊ1L���ס��������ﵽƽ������ʱ�䣺�ף��ң��������������=������ͬ����ƽ��ʱX��Y��ת���ʣ��ף��ң�
���� ��1����ѧƽ�ⳣ��Ϊ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ��
��ͼ1��֪���¶�Խ�ߣ�Z�ĺ���Խ�ͣ�����ƽ�����淴Ӧ�����ƶ����ݴ��жϣ�
ƽ�ⳣ��KֵԽ��Ӧ���еij̶�Խ����Ӧ���ת����Խ�ߣ�
��2����ͼ1��֪��ѹǿԽ�ߣ�Z�ĺ���Խ�ߣ�����ƽ��������Ӧ�����ƶ�����a+b��c����Ϊ��ѹ������ѹǿ���䣬�������п죻
�ҵ�ЧΪ��ѹ��ѹǿ����ƽ���������С�ķ����ƶ�����������Ӧ�ƶ���ת��������
��� �⣺��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK=$\frac{c{\;}^{c}��Z��}{c{\;}^{a}��X��•c{\;}^{b}��Y��}$��
��ͼ1��֪���¶�Խ�ߣ�Z�ĺ���Խ�ͣ�����ƽ�����淴Ӧ�����ƶ���Kֵ��С��
ƽ�ⳣ��KֵԽ��Ӧ���еij̶�Խ����Ӧ���ת����Խ�ߣ���ѡAB��
�ʴ�Ϊ��$\frac{c{\;}^{c}��Z��}{c{\;}^{a}��X��•c{\;}^{b}��Y��}$����С��AB��
��2����ͼ1��֪��ѹǿԽ�ߣ�Z�ĺ���Խ�ߣ�����ƽ��������Ӧ�����ƶ�����a+b��c����Ϊ��ѹ������ѹǿ���䣬�������п죬����ƽ����ʱ��ף��ң�
�ҵ�ЧΪ��ѹ��ѹǿ����ƽ���������С�ķ����ƶ�����������Ӧ�ƶ���ת�����������Լף��ң�
�ʴ�Ϊ����������
���� ������Ҫ����ƽ�ⳣ����ƽ���ƶ��ȣ��ۺ��Խϴ��Ѷ��еȣ�ע���Чƽ��˼��Ľ�����

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�| A�� | ��������SO2��Ʒ����Һ | |
| B�� | ��HClͨ��NaAlO2��Һ�� | |
| C�� | ��Fe��NO3��2��Һ�еμ�ϡ���� | |
| D�� | ��̼��������Һ�еμ�����������Һ |
��CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g������H=-574kJ•mol-1
��CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g������H=-1160kJ•mol-1
����˵������ȷ���ǣ�������
| A�� | �ɷ�Ӧ �ٿ���֪��CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O ��l����H��-574 kJ•mol-1 | |
| B�� | ��Ӧ �٢�ת�Ƶĵ�������ͬ | |
| C�� | ���ñ�״����4.48LCH4��ԭNO2��N2���ų�������Ϊ173.4kJ | |
| D�� | ���ñ�״����4.48LCH4��ԭ NO2�� N2������������ת�Ƶĵ�������Ϊ1.60 mol |
| A�� | CH3COONa�TCH3COO-+Na+ | B�� | H2SO4�TH2++SO42- | ||
| C�� | FeCl3�TFe2++3Cl- | D�� | KClO3�TK++3O2-+Cl- |
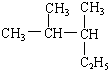 2��3-��������
2��3-�������� ��
��
 ̫���ܵĿ�������������Դ�о���ռ����Ҫ��λ��������̫���ܵ��Ƭ�ڼӹ�ʱ��һ������õ�ﴡ����ء����ȣ�
̫���ܵĿ�������������Դ�о���ռ����Ҫ��λ��������̫���ܵ��Ƭ�ڼӹ�ʱ��һ������õ�ﴡ����ء����ȣ�