��Ŀ����
���ͷ���һ����������Ʒ����ʳƷ�Ļ�ѧ���ɼ�����С�մ���(̼�����)�������е�����������ɡ�ij�о���ѧϰС��Ϊ̽����ͬƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣���������ʵ�顣
��������衿
��1������1����С�մ�ͳ�����ɣ�
����2����С�մ��������ɣ�
����3����________________��ɡ�
�����������̡�
Ϊ̽��ijƷ�Ƶķ��ͷ۵Ļ�ѧ�ɷ֣�ijͬѧ�������ʵ�飬�õ���������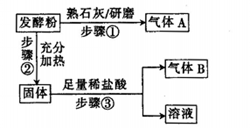
��2���÷��ͷ۵ijɷ�Ϊ________ (�ѧʽ����
��3����һƷ�Ƶķ��ͷ۵Ļ�ѧ��ɿ���Ϊ����2������������ʵ����֤��д��ʵ�鲽�衢Ԥ������ͽ��ۡ�
| ʵ�鲽�� |  Ԥ��������� Ԥ��������� |
| 1��ȡ������Ʒ����ϡ�������Һ�ֳ����� | |
| 2�� _______________________________________ | |
| 3�� ________________________________________ | |
��4����һƷ�Ƶķ��ͷ۵Ļ�ѧ���ΪС�մ��̼����泥�Ϊ̽���仯ѧʽ[��ѧʽ�ɱ�ʾΪnNaHCO3��m NH4HCO3]����ȡ4.05 g�ò�Ʒ���ձ����ܽ������________���100 mL��Һ������Һ��ȡ25.00mL��Һ���μ��������ᣬ���ɵ�����ͨ���������ʯ��ˮ�У����ɵİ�ɫ�������������Ϊ1.25 g����÷��ͷ۵Ļ�ѧʽΪ ��
����Է���������NaHCO3��84 NH4HCO3��79 CaCO3��100��
��17�֣�
��1�����ۺ����� ��2�֣�
��2��NH4HCO3��NaHCO3 ��2�֣�
��3����8�֣�ʵ�鲽��  Ԥ���������
Ԥ���������1��ȡ������Ʒ����ϡ�������Һ�ֳ����� 2���ù���������˿��1�֣�պȡ����һ����Һ�ھƾ����������գ��۲������ɫ��1�֣�____ ����ʻ�ɫ��1�֣����÷��ͷ��к���NaHCO3��1�֣� 3�� ������һ����Һ�еμ�������1�֣�BaCl2��Һ��1�֣���
��������1��2�ξ��ɵ�1�֣�
�Լ�BaCl2�÷֣�Ba��NO3��2��Ba��0H��2���÷֣���д����HCl�ữ���۷֣� �а�ɫ�������ɣ�1�֣�����ϲ���2�Ľ��ۼ������1�֣�����
��4��100mL����ƿ�� 2�֣� 2NaHCO3��3NH4HCO3 (3��)
���������������1�����������֪����ͬƷ�Ƶķ��ͷۿ���С�մ�ͳ�����ɣ�Ҳ������С�մ��������ɣ��������ɳ��ۺ�������ɣ�������֪�ļ���1������2�ƶϼ���3Ϊ���ۺ���������2������NaHCO3��NH4HCO3��Al2(SO4)2?12H2O����Ҫ���ʿ�֪��NH4HCO3����Σ�����ʯ�һ����ĥ���Էų���������������������ʯ����ĥ�����ܷų����壬��AΪNH3�����ͷۼ�һ�����г��ۣ�NH4HCO3�����ּ��Ⱥ���ȫ��Ϊ�����ݳ���NaHCO3���������ȱ�ΪNa2CO3�����CO2��H2O��Na2CO3������������Ӧ���ɶ�����̼���塢NaCl��H2O����Al2(SO4)2?12H2O����ˮ�����Al(OH)3�������϶�������������ɴ��ƶ�BΪCO2���÷��ͷ�һ������С�մ����Լ�Ʒ�Ƶķ��ͷ۵���Ҫ�ɷ���NaHCO3��NH4HCO3����3������ʵ�鷽���в���2�Ľ������ƿ�֪������2���ýྻ�IJ�˿պȡA�е���Һ���پƾ������������գ��������ɫ�ʻ�ɫ��֤����Na+�����ͷ�����NaHCO3�����ڼ���2�Ƿ��ͷ���С�մ��������ɣ�����3�Ľ�����֤�����ͷ���Al2(SO4)2?12H2O�����������ʼ��ṩ�Լ��������ƶϣ�����3�����ʵ�鷽��֤�����ͷ��к���Al3+�����Ӧ��B�Թ�����εμ�0.1mol/LNaOH��Һ���۲쵽��ɫ�������Ȳ�����ɫ����������ܽ⣬֤����Al3+�����ͷ�������������4��������Һ��Ҫ���������������ܽ⡢ת�ơ�ϴ�ӡ����ݵȲ��裬������100 mL��Һ��Ҫ���ܽ⡢��ȴ�����Һ����100mL����ƿ�У�25.00mL������ȫ��Ӧ������CO2+Ca(OH)2=CaCO3��+H2O�����ɫ����Ϊ̼��ƣ�����n=m/M����n(CaCO3)=1.25g��100g/mol=0.0125mol������100mL������Һ�������25.00mL������Һ֮��Ϊ100/25.00����100mL������Һ���������ᷴӦ�ų��Ķ�����̼Ϊ0.0125mol��100/25.00=0.05mol���跢�ͷ���NaHCO3��NH4HCO3�ֱ�Ϊxmol��ymol������m=n��M����84x+79y=4.05����������������NaHCO3+HCl=NaCl+CO2��+H2O��NH4HCO3+HCl=NH4Cl+CO2��+H2O����xmolС�մ���ȫ��Ӧ�ų�xmol������̼���壬ymol̼�������ȫ��Ӧ�ų�ymol������̼����x+y=0.05����������������⣬��x=0.02��y=0.03��˵���÷��ͷ���С�մ��̼����淋����ʵ���֮��Ϊ0.2��0.3=2��3�����Ը÷��ͷ۵���ɿ��Ա�ʾΪ2NaHCO3��3NH4HCO3��
���㣺����̽��ʵ�鷽������Ƽ���ѧ���㣬�漰Ԫ�ػ���������ʡ�������衢���ʵ�鷽����֤���衢��ѧ������ɵĻ�ѧ���������Һ�������������ʵ����ڻ�ѧ����ʽ�����е�Ӧ�õȡ�

ʵ������ȡ���ᶡ����ʵ��װ�������¼ס�������װ�ÿɹ�ѡ�á�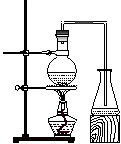
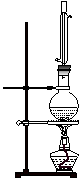
���ף� ���ң�
�Ʊ����ᶡ�����漰���й����ʵ��������ʼ��±�
| | ���� | 1������ | ���ᶡ�� |
| �۵�(��) | 16��6 | ��89��5 | ��73��5 |
| �е�(��) | 117��9 | 117 | 126��3 |
| �ܶ�(g/cm3) | 1��05 | 0��81 | 0��88 |
| ˮ���� | ���� | ���� (9g/100gˮ) | �� |
��1����ȡ���ᶡ����װ��Ӧѡ��___________(��ס����ҡ�)����ѡ��һ��װ�õ�������______________________________________________________________��
��2����ʵ���������г������������ᶡ���⣬���������ɵ��л��������У�д���ṹ��ʽ��________________________________________________________________��
��3��������Ӧ��һ�����淴Ӧ��Ϊ���1�������������ʣ�д�����ֿ��еķ�����
�� ����
��4�����Ʊ����ᶡ�����õĻ�����з��롢�ᴿ���ᶡ��ʱ����Ҫ�����ಽ����������ͼʾ�IJ����У��϶���Ҫ�Ļ�ѧ������________________��ѡ��𰸱�ţ���

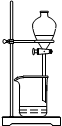


A B C D
��5���л���ķ�������У�������Ҫʹ�÷�Һ©����������ʹ�÷�Һ©��ǰ����_______________��ijͬѧ�ڽ��з�Һ����ʱ��������Һ�����������������ԭ�����Һ©�����������⣬���� ��
̼�����׳ƴ������;�ܹ㡣ʵ�����У���̼����狀ͱ���ʳ��ˮ���Ƶô���������ڲ�ͬ�¶��µ��ܽ�ȼ�����
  �¶ȡ� �¶ȡ��ܽ�� ���� g/100gˮ | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
| NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 37.8 |
| NH4HCO3 | 15.8 | 21.0 | 27.0 | | | | |
| NaHCO3 | 8.2 | 9.6 | 11.1 | 12.7 | 14.4 | 16.4 | |
| NH4Cl | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.2 | 60.2 |
ʵ�鲽��
�����뾫�ƣ��ٴ���(��Ca2+��Mg2+��SO42��)�ܽ⣻�ڼ�������NaOH��Na2CO3��Һ����У��۹��ˣ��ܼ��������pH��7��
��ת�����ٽ����ƺ��ʳ����Һ�¶ȿ�����30~35��֮�䣻�ڲ��Ͻ����£�������ϸ��̼����泥����£������Сʱ���ھ��ã� a �� b ���۵õ�NaHCO3���塣
���ƴ�����õ�NaHCO3�����������У��ھƾ��������գ���ȴ�����£����õ����
���������գ�
��1���������뾫�ơ��ɳ�ȥ�Ĵ����е����������� ��
��2����ת���������ӷ���ʽ�� ��
��3����ת���������У��¶ȿ�����30~35��֮��ļ��ȷ�ʽ�� ��Ϊʲô�¶ȿ�����30~35��֮�䣿 ��
��4��a��b���IJ����ֱ��� �� ��
��5��ʵ�����ƵõĴ������NaCl�����ܺ�����NaHCO3��Ϊ�ⶨ����Ĵ��ȣ��õ�����ƽȷ��ȡ��ƷG�ˣ����������ƿ������������ˮ�ܽ⣬�μ�2�η�̪����c mol/L�ı�����ζ�����Һ��dz��ɫ�����ɫ�Ұ���Ӳ��䣬�ζ������������������������������ΪV1 mL����ʱ�����ķ�ӦΪ��CO32�� + H+ ��HCO3��
����Ʒ��̼���������ٷֺ����ı���ʽ�� ��
������ƿ��Һ�м����μ�2�μ��ȣ���ͬŨ�ȵ���������ζ����յ㣬������������ΪV2mL���ζ��յ�ʱ��Һ��ɫ�ı仯�� ������ʵ�����ݣ�����ж���Ʒ����NaHCO3 ��
�ִ�п��Ʒ�ӹ���ҵ���յķ���������ZnO��FeO��Fe2O3��CuO��Al2O3�����ʣ�����ȡ����п���������£�
�й�����������ȫ������pH���±���
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 6.7 | 8.0 |
��l������������У�Ҫ���пԪ�صĽ����ʣ����Բ�ȡ ��ʩ��
��2�����������жദ�漰�����ˡ���ʵ�����й��˲�����Ҫʹ�õIJ��������� ��
��3���ڡ�����I�������У�����Һ����pH=4��Ŀ���� ���ڡ�����II������Һ��pHԼΪ6����˲�����ʱ�����к��� ��
��4���ڡ�̼���ϳɡ��У����ɵIJ���֮һΪ��ʽ̼��п[Zn2��OH��2CO3]��ͬʱ�ų�CO2����д���÷�Ӧ�Ļ�ѧ����ʽ ��
��5������Һ����ȡNaNO3����IJ�������Ϊ ��
��6����ʵ�������ϴ�ӹ��˳��ļ�ʽ̼��п�� ��
NaCl��NaClO�����������¿ɷ�����Ӧ��ClO��+Cl��+2H+ = Cl2��+H2O��ijѧϰС�����о�����Һ(��Ҫ�ɷ�ΪNaCl��NaClO)�ı��������
��1��������Һ��NaClO�����տ����е�CO2����NaHCO3��HClO�����ʡ�д����ѧ��Ӧ����ʽ ��
��2��ȡ��������Һ�����Թ��У���������һ��Ũ�ȵ����ᣬ������ų���ͨ������װ�ü�������ijɷֿ����ж�����Һ�Ƿ���ʡ�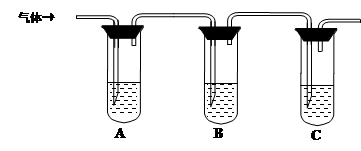
��ѡ�Լ���98%Ũ���ᡢ1%Ʒ����Һ��1.0 mol��L��1 KI-������Һ��1.0 mol��L��1NaOH������ʯ��ˮ������NaCl��Һ
���������ʵ�鷽����
| �����Լ� | Ԥ������ͽ��� |
| �Թ�A�м������� �� �Թ�B�м�1%Ʒ����Һ�� �Թ�C�мӢ� �� | ��A����Һ����ɫ��B����Һ����ɫ��C����Һ����ǡ�������Һ���ֱ��ʣ� �� ������Һδ���ʣ� �� ������Һ��ȫ���ʡ� |
��3���õζ����ⶨ����Һ��NaClO��Ũ�ȡ�ʵ�鲽�����£�
����ȡ 25.00mL����Һ������ƿ�У����������a mol��L��1 Na2SO3��Һb mL��
�ڵζ���������c mol��L��1������KMnO4��Һװ�� ������ʽ���ʽ���ζ����У�KMnO4��ʣ���Na2SO3������Ӧ������Һ����ɫ���dz��ɫ���ұ��ְ�����ں�ɫ����ʱ��ֹͣ�ζ�����¼���ݡ��ظ��ζ�ʵ��2�Σ�ƽ����������KMnO4��Һv mL��
�ζ��������漰�ķ�Ӧ�У�NaClO + Na2SO3 = NaCl+ Na2SO4 ��
2KMnO4 + 5Na2SO3+ 3H2SO4 = K2SO4 + 2MnSO4 + 5Na2SO4 + 3H2O
�ۼ��㡣����Һ��NaClO��Ũ��Ϊ mol��L��1���ú�a��b��c��v�Ĵ���ʽ��ʾ����
�������ȣ�ClO2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�������������һ�ֻ���ɫ�����壬������ˮ��ʵ���ҿ���NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ�����Ʊ�ClO2�����������£�
��1��д�����ʱ������Ӧ�Ļ�ѧ����ʽ�� ��
��2����ȥClO2�е�NH3��ѡ�õ��Լ��� ��������ĸ��
| A������ʳ��ˮ | B����ʯ�� | C��Ũ���� | D��ˮ |
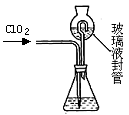
��װ���в���Һ��ܵ������� �� ��
����д��������������������⻯����Һ��Ӧ�����ӷ���ʽ �� ��
�۵ζ��յ�������ǣ� ��
�ܲ��ͨ��ClO2������m(ClO2)= �����ú�c��V�Ĵ���ʽ��ʾ������֪��ClO2����Է�������Ϊ67.5��
��4�����ʵ����ȷ����ҺX�ijɷ֣��벹�����ʵ�鲽�������
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �� | | ��ҺX�к���Na+ |
| �� | | ��ҺX�к���Cl�� |
�����±��ṩ��������ҩƷ�����ܴﵽ��Ӧʵ��Ŀ�ĵ���
| ��� | ���� | ҩƷ | ʵ��Ŀ�� |
| A | ������ƽ�������룩��250mL����ƿ����Ͳ���ձ���ҩ�ס������� | NaOH���塢����ˮ | ����250mLһ�����ʵ���Ũ�ȵ�NaOH��Һ |
| B | ��Һ©������ƿ�����ܼ���Ƥ�� | ϡ���ᡢ̼���ơ���������Һ | ֤���ǽ����ԣ�S��C��Si |
| C | ��ʽ�ζ��ܡ���ʽ�ζ��ܡ���ͷ�ιܡ�����̨�������У�����ƿ | ��֪Ũ�ȵ�NaOH��Һ���������ᡢ����ˮ����ֽ | �ⶨϡ��������ʵ���Ũ�� |
| D | ����̨�������У����ƾ��ơ����Թܡ�����ƿ�����ܼ���Ƥ�� | �Ȼ�� | ��ȡ���� |
��ʵ����������м������ͭ��ϡ����Ϊԭ���Ʊ�ͭ������������;����
��1��Fe H2
H2 Cu
Cu
��2��CuO CuSO4
CuSO4 Cu
Cu
���������ַ����Ƶõ�����ͭ���������й�˵������ʵ��������ǣ� ��
| A����������������ͬ |
| B�����������������ͬ |
| C����������������������ͬ |
| D����������ͭ��������ͬ |

 ��������ͼװ�ü���÷�Ӧ��������
��������ͼװ�ü���÷�Ӧ��������
