��Ŀ����
�����ֲ�ͬ�����ȵ�����ͭ��̿�۵Ļ������Ʒ����������.�ס��ҡ�����ͬѧ��ȡһ����Ʒ����ǿ�ȳ�ַ�Ӧ���ⶨ����Ʒ������ͭ����.(1)��ȡ��Ʒ��ǿ�ȣ������ù���Ϊ����ͭ����������������ϡ�������ȣ�����1.12L����(��״��)������Ʒ��������ͭ������Ϊ________g.
(2)��ȡ��Ʒ��a gǿ�ȣ����ɵ����岻��ʹ�����ʯ��ˮ�����.�ٽ���Ӧ��Ĺ�����������ϡ�����ȣ���ַ�Ӧ����b g����ʣ�࣬��ʣ�����Ļ�ѧʽΪ________________.��Ʒ��������ͭ����Ϊ________________g(�Ժ�a��b�Ĵ���ʽ��ʾ).
(3)��������Ʒ��ǿ�Ⱥ�ʣ��Ĺ��壬������ԭ��Ʒ��С��c g�����ù���Ϊ����ͭ������Ʒ��������ͭ���ʵ���(n)��ȡֵ��ΧΪ________________.
�𰸣�
������
��ʾ��
������
| ��1��6.0 ��2�� ��3��
|
��ʾ��
| (1)3Cu+8HNO3(ϡ)=3Cu(NO3)2+2NO��+4H2O��NOΪ1.12L����0.05mol������CuΪ0.075mol����������ͭΪ0.075��80��6.0g
(2) ���ɵ����岻��ʹ�����ʯ��ˮ�����������ΪCO������C̼�۹���������ϡ���ᷴӦ�Ĺ���ΪC��Ϊ b g�����Է�Ӧ��������ͭ��̼��Ϊa��b g��CuO��C��Cu��CO����a��b��g�ж��ߵ����ʵ������. (3)ʣ�����Ϊͭ����̼��ȫ��Ӧ�ˣ���Ӧ���ɵ�����ΪCO��CO2������߶���.
|

��ϰ��ϵ�д�
�����Ŀ



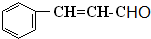



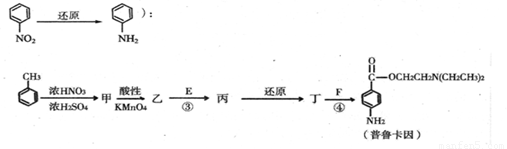
 ����һ�֣�
����һ�֣�
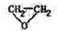
 ����
����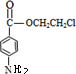
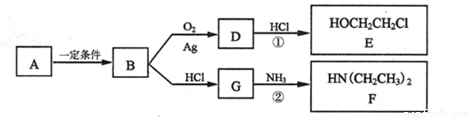
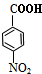 +HOCH2CH2Cl
+HOCH2CH2Cl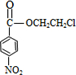 +H2O
+H2O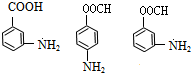
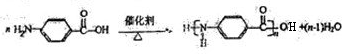

 )��
)��