��Ŀ����
��2012?ʯ��ׯһģ������ѧһѡ��5���л���ѧ������
��̼���⡢������Ԫ����ɵ��л���A������Է�������Ϊ118��A�к������������Ϊ8.5%����������ԭ�Ӹ���Ϊ̼ԭ�Ӹ�����2����
��1��A�ķ���ʽΪ
��2��A�к�����������һ���������������ظ������Һ��Ӧ��������B��B�ĺ˴Ź���������ʾ�������壬�����֮��Ϊ1��3��
��B�Ľṹ��ʽΪ

��A�ܷ����ķ�ӦΪ
a���ӳɷ�Ӧ b����ȥ��Ӧ c��������Ӧ d�����۷�Ӧ
��3����������������A��ͬ���칹����

����A������ͬ�Ĺ����Ţ�ֻ����һ����
��4��D��A��һ�ֺ�������ͬ���칹�壬D������������ˮ������M��N���Ҷ�����Է����������16��M�������ֲ�ͬ��ѧ�������⣮д��D�����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ��
��5��B���Ҷ�����һ�������·�����Ӧ��������һ�ֺ�����Ԫ���������÷�Ӧ�Ļ�ѧ����ʽΪ
 ��
��
��̼���⡢������Ԫ����ɵ��л���A������Է�������Ϊ118��A�к������������Ϊ8.5%����������ԭ�Ӹ���Ϊ̼ԭ�Ӹ�����2����
��1��A�ķ���ʽΪ
C5H10O3
C5H10O3
����2��A�к�����������һ���������������ظ������Һ��Ӧ��������B��B�ĺ˴Ź���������ʾ�������壬�����֮��Ϊ1��3��
��B�Ľṹ��ʽΪ


��A�ܷ����ķ�ӦΪ
cd
cd
����ѡ����ĸ����a���ӳɷ�Ӧ b����ȥ��Ӧ c��������Ӧ d�����۷�Ӧ
��3����������������A��ͬ���칹����
6
6
�֣�д����������һ��ͬ���칹��Ľṹ��ʽ

����A������ͬ�Ĺ����Ţ�ֻ����һ����
��4��D��A��һ�ֺ�������ͬ���칹�壬D������������ˮ������M��N���Ҷ�����Է����������16��M�������ֲ�ͬ��ѧ�������⣮д��D�����������·���ˮ�ⷴӦ�Ļ�ѧ����ʽ��
CH3COOCH2CH2CH2OH+H2O
CH3COOH+HOCH2CH2CH2OH
| Ũ���� |
| �� |
CH3COOCH2CH2CH2OH+H2O
CH3COOH+HOCH2CH2CH2OH
| Ũ���� |
| �� |
��5��B���Ҷ�����һ�������·�����Ӧ��������һ�ֺ�����Ԫ���������÷�Ӧ�Ļ�ѧ����ʽΪ


�������л���A��Է�������Ϊ118��A�к������������Ϊ8.5%����Hԭ����Ϊ��
=10����������ԭ�Ӹ���Ϊ̼ԭ�Ӹ�����2��������Cԭ����Ϊ5����Oԭ����Ϊ��
=3������A�ķ���ʽΪC5H10O3������A�Ĺ������ж������ʣ�
��1��A�ķ���ʽΪC5H10O3��
��2���ٸ���A�ķ���ʽΪC5H10O3�������ܱ������ظ������Һ��������A����-OH������һ��-COOH��Ȼ��ȷ����ṹ��ʽ��Ȼ��ȷ��B�Ľṹ��
�ڸ���A�еĹ����ŷ��������ʣ�
��3��A�ķ���ʽΪC5H10O3�������к���-OH������һ��-COOH��ֻ����һ�������ݴ�д�����ܵĽṹ��ʽ��
��4��D��A��һ�ֺ�������ͬ���칹�壬����������Ϣ�ƶ���ṹ��ʽ����ˮ�ⷽ�̣�
��5��B�����Ȼ����봼����������Ӧ��
| 118��0.085 |
| 1 |
| 118-10-12��5 |
| 16 |
��1��A�ķ���ʽΪC5H10O3��
��2���ٸ���A�ķ���ʽΪC5H10O3�������ܱ������ظ������Һ��������A����-OH������һ��-COOH��Ȼ��ȷ����ṹ��ʽ��Ȼ��ȷ��B�Ľṹ��
�ڸ���A�еĹ����ŷ��������ʣ�
��3��A�ķ���ʽΪC5H10O3�������к���-OH������һ��-COOH��ֻ����һ�������ݴ�д�����ܵĽṹ��ʽ��
��4��D��A��һ�ֺ�������ͬ���칹�壬����������Ϣ�ƶ���ṹ��ʽ����ˮ�ⷽ�̣�
��5��B�����Ȼ����봼����������Ӧ��
����⣺�л���A��Է�������Ϊ118��A�к������������Ϊ8.5%����Hԭ����Ϊ��
=10����������ԭ�Ӹ���Ϊ̼ԭ�Ӹ�����2��������Cԭ����Ϊ5����Oԭ����Ϊ��
=3������A�ķ���ʽΪC5H10O3������A�Ĺ������ж������ʣ�
��1���������ƶϿ�֪��A�ķ���ʽΪC5H10O3���ʴ�Ϊ��C5H10O3��
��2������֪A�ķ���ʽΪC5H10O3��˵�������к���1�������ͼ��������ܱ������ظ������Һ��������A����-OH��һ��-COOH����֪A�к���������������A�Ľṹ��ʽΪ��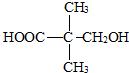 ��A��������������B����B�ĽṹΪ��
��A��������������B����B�ĽṹΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
����֪A�еĹ�����Ϊ-OH��-COOH����A�ܷ���������Ӧ �����۷�Ӧ���ʴ�Ϊ��cd��
��3��A�ķ���ʽΪC5H10O3�������к���-OH������һ��-COOH��ֻ����һ����������ܵĽṹ��ʽΪ��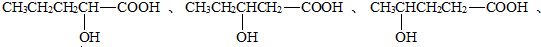
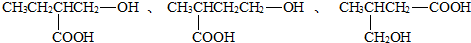
���Կ��ܵĽṹ��ʽ��6�֣�
�ʴ�Ϊ��6�� ��
��
��4��D��A��һ�ֺ�������ͬ���칹�壬D������������ˮ������M��N���Ҷ�����Է����������16��M�������ֲ�ͬ��ѧ�������⣬����N�ķ�����Ϊx����M �ķ�����Ϊx+16������118+18=x+x+16 ��x=60����NΪCH3COOH��MΪHOCH2CH2CH2OH������DΪCH3COOCH2CH2CH2OH����ˮ�ⷽ��Ϊ��CH3COOCH2CH2CH2OH+H2O
CH3COOH+HOCH2CH2CH2OH���ʴ�Ϊ��CH3COOCH2CH2CH2OH+H2O
CH3COOH+HOCH2CH2CH2OH��
��5��B�����Ȼ����봼����������Ӧ�� ���Ҷ�����һ�������·�����Ӧ��������һ�ֺ�����Ԫ���������÷�Ӧ�Ļ�ѧ����ʽΪ��
���Ҷ�����һ�������·�����Ӧ��������һ�ֺ�����Ԫ���������÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
| 118��0.085 |
| 1 |
| 118-10-12��5 |
| 16 |
��1���������ƶϿ�֪��A�ķ���ʽΪC5H10O3���ʴ�Ϊ��C5H10O3��
��2������֪A�ķ���ʽΪC5H10O3��˵�������к���1�������ͼ��������ܱ������ظ������Һ��������A����-OH��һ��-COOH����֪A�к���������������A�Ľṹ��ʽΪ��
 ��A��������������B����B�ĽṹΪ��
��A��������������B����B�ĽṹΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
������֪A�еĹ�����Ϊ-OH��-COOH����A�ܷ���������Ӧ �����۷�Ӧ���ʴ�Ϊ��cd��
��3��A�ķ���ʽΪC5H10O3�������к���-OH������һ��-COOH��ֻ����һ����������ܵĽṹ��ʽΪ��
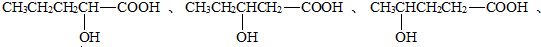
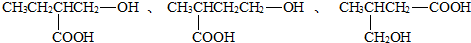
���Կ��ܵĽṹ��ʽ��6�֣�
�ʴ�Ϊ��6��
 ��
����4��D��A��һ�ֺ�������ͬ���칹�壬D������������ˮ������M��N���Ҷ�����Է����������16��M�������ֲ�ͬ��ѧ�������⣬����N�ķ�����Ϊx����M �ķ�����Ϊx+16������118+18=x+x+16 ��x=60����NΪCH3COOH��MΪHOCH2CH2CH2OH������DΪCH3COOCH2CH2CH2OH����ˮ�ⷽ��Ϊ��CH3COOCH2CH2CH2OH+H2O
| Ũ���� |
| �� |
| Ũ���� |
| �� |
��5��B�����Ȼ����봼����������Ӧ��
 ���Ҷ�����һ�������·�����Ӧ��������һ�ֺ�����Ԫ���������÷�Ӧ�Ļ�ѧ����ʽΪ��
���Ҷ�����һ�������·�����Ӧ��������һ�ֺ�����Ԫ���������÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
���ʴ�Ϊ��
 ��
�����������⿼���л�����ƶϣ���Ҫѧ���Ը������Ϣ�������ã��ܽϺõĿ���ѧ�����Ķ���������ѧ��������Ŀ�ѶȽϴ��ۺ��Խ�ǿ��

��ϰ��ϵ�д�
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
�����Ŀ
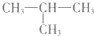 ��2012?ʯ��ׯһģ��2-�����飨��ͼ���Ķ��ȴ��ﹲ�У������������칹����������
��2012?ʯ��ׯһģ��2-�����飨��ͼ���Ķ��ȴ��ﹲ�У������������칹����������