��Ŀ����
����Ŀ���� X��Y��Z ����Ԫ�أ�X ���л��������бغ���Ԫ�أ� Y �ǵؿ��ﺬ������Ԫ�أ�Z ���� �������Ԫ�ء�X��Y��Z ����Ԫ����ɵ��л��� M �ܱ����Ը�������������� N��Ϊ�˲ⶨ�л��� M �Ľṹ��������ʵ�飺
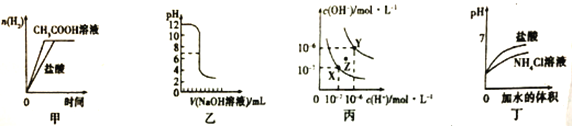
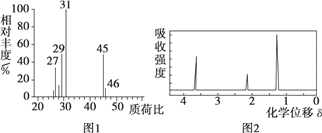
���� 4.6 g �л��� M ��ȫȼ�գ�������� 0.2mol CO2 �� 5.4 g ˮ�� ���������Ǽ���л��� M���õ���ͼ 1 ��ʾ������ͼ�� ���ú˴Ź����Ǵ����л��� M���õ���ͼ 2 ��ʾͼ�ס�
�Իش��������⣺
��1��M �Ľṹ��ʽ��___________________��N �к��еĹ����ŵĽṹ��ʽΪ_____________��
��2��д�� M ��ͭ�������Ҽ���������������������Ӧ�Ļ�ѧ����ʽ___________________��
��3��д�� M �� N ��Ũ H2SO4���������·�����Ӧ�Ļ�ѧ����ʽ___________________��
���𰸡� CH3CH2OH ��COOH 2CH3CH2OH��O2![]() 2CH3CHO��2H2O CH3COOH��CH3 CH2OH
2CH3CHO��2H2O CH3COOH��CH3 CH2OH![]() CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O
��������X��Y��Z ����Ԫ�أ�X���л��������бغ���Ԫ�أ���XΪ̼Ԫ�أ�Y�ǵؿ��ﺬ������Ԫ�أ���YΪ��Ԫ�أ�Z �����������Ԫ�أ���ZΪ��Ԫ�ء�
�������Ǽ���л��� M���õ���ͼ 1 ��ʾ������ͼ������Է�������Ϊ46��ͨ�����㣬������̼�����ʵ���Ϊ0.2mol��ˮ�����ʵ���Ϊ![]() =0.3mol�������л����к���ԭ�ӵ����ʵ���=
=0.3mol�������л����к���ԭ�ӵ����ʵ���=![]() =0.1mol������̼������ԭ�Ӹ�����Ϊ2:6:1��������Է���������������ѧʽΪC2H6O�����ݺ˴Ź�������������������ԭ�ӣ�����Ϊ3:2:1������M�Ľṹ��ʽΪ��CH3CH2OH��M �ܱ����Ը�������������� N��NΪCH3COOH����1��M �Ľṹ��ʽ��CH3CH2OH��N ΪCH3COOH�����еĹ����ŵĽṹ��ʽΪ-COOH����2��CH3CH2OH��ͭ�������Ҽ���������������������Ӧ����CH3CHO����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH��O2
=0.1mol������̼������ԭ�Ӹ�����Ϊ2:6:1��������Է���������������ѧʽΪC2H6O�����ݺ˴Ź�������������������ԭ�ӣ�����Ϊ3:2:1������M�Ľṹ��ʽΪ��CH3CH2OH��M �ܱ����Ը�������������� N��NΪCH3COOH����1��M �Ľṹ��ʽ��CH3CH2OH��N ΪCH3COOH�����еĹ����ŵĽṹ��ʽΪ-COOH����2��CH3CH2OH��ͭ�������Ҽ���������������������Ӧ����CH3CHO����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH��O2![]() 2CH3CHO��2H2O����3��CH3CH2OH �� CH3COOH��Ũ H2SO4���������·�����Ӧ�Ļ�ѧ����ʽΪ��CH3COOH��CH3 CH2OH
2CH3CHO��2H2O����3��CH3CH2OH �� CH3COOH��Ũ H2SO4���������·�����Ӧ�Ļ�ѧ����ʽΪ��CH3COOH��CH3 CH2OH![]() CH3COOCH2CH3��H2O��
CH3COOCH2CH3��H2O��

 ��������������������ϵ�д�
��������������������ϵ�д�