��Ŀ����
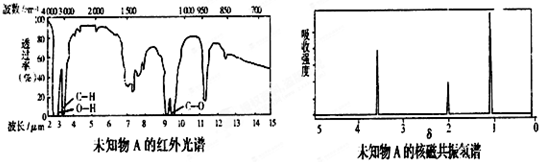
��֪���л���A��B��C��̼���⡢����Ԫ�����ʵ���֮�Ⱦ�Ϊ1��2��1�����Ƕ�����������Ӧ���������ܷ���ˮ�ⷴӦ��B1��B2��B��ͬ���칹�壮��֪��A�ڳ�����Ϊ���壬A��C6H5OH Z(�߷��ӻ�����)��B1��16.6��������Ϊ��״���壬B1��Na2CO3
Z(�߷��ӻ�����)��B1��16.6��������Ϊ��״���壬B1��Na2CO3 ��B2Ϊ��ɫҺ�壬Ҳ�ܷ���������Ӧ������Na��Ӧ��1mol C��ȫȼ����Ҫ3mol�������Իش�
��B2Ϊ��ɫҺ�壬Ҳ�ܷ���������Ӧ������Na��Ӧ��1mol C��ȫȼ����Ҫ3mol�������Իش�
(1)B2��������________���ṹ��ʽ��AΪ________��BΪ________��
(2)д��X��Y�Ļ�ѧ����ʽ��________________________________________��
(3)C�Ľṹ��ʽΪ________����C��Ϊͬ���칹�壬�����������������Ľṹ��ʽ��________��________��
�𰸣�
������
������
|
(1)�������,HCHO,HOCH2CHO (2)CH3COONa��NaOH (3) |

��ϰ��ϵ�д�
�����Ŀ
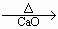 CH4����Na2CO3
CH4����Na2CO3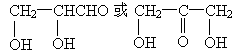 ,HOCH2COOCH3,CH3COOCH2OH
,HOCH2COOCH3,CH3COOCH2OH