��Ŀ����
��19�֣�
��.��̼�����缫���������ˮ��Һ����Na2SO4��Һ��AgNO3��Һ��KCl��Һ��CuCl2��Һ��ͨ����ͬ����ʱ�������������������ʵ�����ͬ���ǣ�����ţ�_________��ͨ����ͬ����ʱ�����������������ʵ����ɶൽ�ٵ�����˳���ǣ�����ţ�__________��
��.��ĿǰΪֹ���ɻ�ѧ��ת������ܻ������Ȼ������ʹ�õ�����Ҫ����Դ��
��1����ѧ��Ӧ�зų����������ʱ䣬��H���뷴Ӧ���������ļ���(E)�йء�
��֪��H2(g)��Cl2(g)��2HCl(g) ��H����185 KJ/mol
E���ȣ��ȣ���436 KJ/mol�� E��Cl��Cl����247 KJ/mol����E��H��Cl����_____
��2����֪Fe2O3(s)��3CO(g)��2Fe(s)��3CO2 (g) ��H����25 KJ/mol
(g) ��H����25 KJ/mol
3Fe2O3(s)��CO(g)��2Fe3O4(s)��CO2(g) ��H����47 KJ/mol
Fe3O4(s)��CO(g)��3FeO(s)��CO2(g) ��H����19 KJ/mol
��д��CO��ԭFeO���Ȼ�ѧ����ʽ��_________________________________��
��3����ͼ��ʾ��װ�ã�
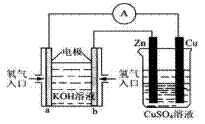
��װ����Cu��Ϊ_____������ͭƬ�������仯��12.8gʱ��a�������ĵ�O2�ڱ�״���µ����Ϊ_______L��
��.��̼�����缫���������ˮ��Һ����Na2SO4��Һ��AgNO3��Һ��KCl��Һ��CuCl2��Һ��ͨ����ͬ����ʱ�������������������ʵ�����ͬ���ǣ�����ţ�_________��ͨ����ͬ����ʱ�����������������ʵ����ɶൽ�ٵ�����˳���ǣ�����ţ�__________��
��.��ĿǰΪֹ���ɻ�ѧ��ת������ܻ������Ȼ������ʹ�õ�����Ҫ����Դ��
��1����ѧ��Ӧ�зų����������ʱ䣬��H���뷴Ӧ���������ļ���(E)�йء�
��֪��H2(g)��Cl2(g)��2HCl(g) ��H����185 KJ/mol
E���ȣ��ȣ���436 KJ/mol�� E��Cl��Cl����247 KJ/mol����E��H��Cl����_____
��2����֪Fe2O3(s)��3CO(g)��2Fe(s)��3CO2
 (g) ��H����25 KJ/mol
(g) ��H����25 KJ/mol 3Fe2O3(s)��CO(g)��2Fe3O4(s)��CO2(g) ��H����47 KJ/mol
Fe3O4(s)��CO(g)��3FeO(s)��CO2(g) ��H����19 KJ/mol
��д��CO��ԭFeO���Ȼ�ѧ����ʽ��_________________________________��
��3����ͼ��ʾ��װ�ã�

��װ����Cu��Ϊ_____������ͭƬ�������仯��12.8gʱ��a�������ĵ�O2�ڱ�״���µ����Ϊ_______L��
��19�֣��٢ۣ�2�֣� �ۢ٢ܢ� (4��)
��1�� 434KJ/mol (4��)
(2) FeO(s)��CO(g)��Fe(s)��C O2(g) ��H����11 KJ/mol (4��)
O2(g) ��H����11 KJ/mol (4��)
(3) ����2�֣� 2.24L (3��)
��1�� 434KJ/mol (4��)
(2) FeO(s)��CO(g)��Fe(s)��C
 O2(g) ��H����11 KJ/mol (4��)
O2(g) ��H����11 KJ/mol (4��)(3) ����2�֣� 2.24L (3��)
��

��ϰ��ϵ�д�
�����Ŀ
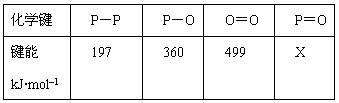
 2SO3( g ) ���� H =-QkJ��mol-1�����������·ֱ���������ͷ�Ӧ�ų������� ( Q)���±����У������������ݣ�����������ȷ���ǣ� ��
2SO3( g ) ���� H =-QkJ��mol-1�����������·ֱ���������ͷ�Ӧ�ų������� ( Q)���±����У������������ݣ�����������ȷ���ǣ� ��

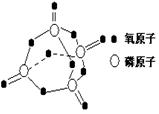
 ����֪����1molN��N����167kJ����������1mol N��N�ų�942kJ����������������Ϣ�����ݣ�����N2��������1mol��̬N4�ġ�HΪ�� ��
����֪����1molN��N����167kJ����������1mol N��N�ų�942kJ����������������Ϣ�����ݣ�����N2��������1mol��̬N4�ġ�HΪ�� ��