��Ŀ����
5�� H��C��N��O��Mg��Al��Si��S��Cu����ѧ��ѧ�г�����Ԫ�أ����������ش�����ЩԪ���йص����⣺
H��C��N��O��Mg��Al��Si��S��Cu����ѧ��ѧ�г�����Ԫ�أ����������ش�����ЩԪ���йص����⣺��1��S�����ӵ�ԭ�ӽṹʾ��ͼΪ
 ��
����2��H2O2��ʵ���г��õġ���ɫ����������1molH218O2��������Ϊ20moL��
��3������Ԫ�������ɣ�̼�ķǽ�����ǿ�ڹ裬����һ����ѧ��Ӧ����ʽ��ʾCO2+Na2SiO3+H2O=H2SiO3��+Na2CO3��
��4����ĩ״��Si3N4�Կ�����ˮ�����ȶ���������ĩ״��Si3N4����������þ��һ�������µ��ܱ��������ȴ��������Եõ��Կ�����ˮ�����ᶼ�൱�ȶ��Ĺ�����ϣ�ͬʱ�������ɶ�ˮ���ȶ���Mg3N2���ȴ������ȥ���������δ��Ӧ��Si3N4��MgO��Mg3N2�ķ����Ǽ�����ϡ�������ˣ�
��5��ijͭ���Ͻ�������ϡ������ȫ�ܽ⣬�õ���״����NO 11.2L��������Һ�м��������ˮ����ַ�Ӧ����ˣ���Һ����ɫ����ͭ��������-[Cu��NH3��4]2+������������Ϊ7.8g����Ͻ������Ϊ41.1g��
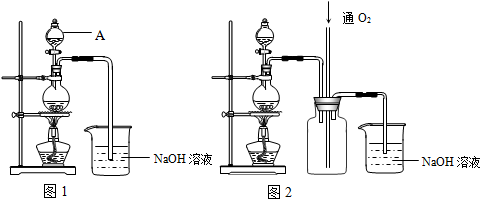
��6����NO��NO2��ɵĻ������ͨ����ͼ��ʾ��װ���У�������֤Ũ����������Ա�ϡ�����������ǿ����ͨ��������֮ǰ����ͨ��һ��ʱ���N2����
��֪��
��i��Ũ�����ܽ�NO������NO2����ϡ���������NO��
��ii��NaOH��Һ��NO2�ܷ�����Ӧ���������κ�ˮ��NaOH��Һ��NO����Ӧ��
a��װ�âڡ��ۡ�����ʢ�ŵ�ҩƷ������ϡ���ᡢˮ��Ũ���
b���ܹ�˵��ʵ���ѳɹ���������װ�â�Һ���Ϸ�������Ϊ��ɫ��װ�â�Һ���Ϸ�����������ɫ��Ϊ����ɫ��
���� ��1�������ӵĽṹʾ��ͼΪ ��
��
��2��1��H218O2��������Ϊ��18-8����2=20���ݴ˼��㣻
��3������Ԫ�طǽ�����Խǿ����Ӧ��ۺ����������Խǿ����������ǿ��������ķ���˵��̼�ķǽ�����ǿ�ڹ裻
��4���ȴ�����õ��Կ�����ˮ�����ᶼ�൱�ȶ��Ĺ�����ϣ�ͬʱ�������ɶ�ˮ���ȶ���Mg3N2����MgO��Mg��OH��2�������ᣬ�ʳ�ȥ�ȶ���������е�Si3N4��MgO��Mg3N2�ɼ�����ϡ�������ˣ�
��5��ͭ���Ͻ�������ϡ���ᷢ��������ԭ��Ӧ��ת��������ͭ������������������õ����ӱ���ԭΪNO����Ӧ�����Һ�м�������İ�ˮ��ͭ���ӷ�Ӧ����Cu��NH3��42+����������ȫ��Ӧ���������������������������غ��������ԭ��Ӧ�е��ӵ�ʧ�غ������
��6������Ũ����������NO��ϡ���������NO��������֤����װ�â���ˮ�������dz�ȥ��������е�NO2������ô�����NO��NaOH������������������ʢ��е�Һ��ΪŨ���ᣬ����Ϊϡ���ᣬװ�âٿ����ռ�NO��װ�â�Һ���Ϸ�������Ϊ��ɫ��װ�â�Һ���Ϸ�����������ɫ��Ϊ����ɫ��˵��Ũ����������Ա�ϡ����ǿ��
��� �⣺��1�������ӵĽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2����Ϊ1��H218O2��������Ϊ��18-8����2=20������1molH218O2��������Ϊ20moL���ʴ�Ϊ��20moL��
��3��Ԫ�طǽ�����Խǿ����Ӧ��ۺ����������Խǿ����������ǿ��������ķ���˵��̼�ķǽ�����ǿ�ڹ裬����ʽ��CO2+Na2SiO3+H2O=H2SiO3��+Na2CO3���ʴ�Ϊ��CO2+Na2SiO3+H2O=H2SiO3��+Na2CO3��
��4���ȴ�����õ��Կ�����ˮ�����ᶼ�൱�ȶ��Ĺ�����ϣ�ͬʱ�������ɶ�ˮ���ȶ���Mg3N2��Mg3N2�����ᷴӦ����MgCl2��NH4Cl����MgO��Mg��OH��2�������ᣬ�ʳ�ȥ�ȶ���������е�Si3N4��MgO��Mg3N2�ɼ�����ϡ�������ˣ��ʴ�Ϊ��������ϡ�������ˣ�
��5����Ͻ���ͭ�����ʵ���Ϊxmol���������ʵ���Ϊymol����Ӧ��ͭʧȥ���ӱ�ɶ���ͭ���ӣ���ʧȥ���ӱ�������ӣ������е�ԭ����+5�۽�ΪNO�е�+2�ۣ�����������ԭ��Ӧ�е�ʧ������ȵĹ�����
2x+3y=$\frac{11.2L}{22.4L/mol}$����5-2����
��Ӧ��������ȫ����Ϊ������������ԭ�Ӹ����غ�ã�
Al��Al��OH��3
1mol 78g
y 7.8g
y=0.1mol
����2x+3y=$\frac{11.2L}{22.4L/mol}$����5-2��
��ã�x=0.6mol
y=0.1mol
�Ͻ������=m��Mg��+m��Al��=0.6mol��64g/mol+0.1mol��27g/mol=41.1g
�ʴ�Ϊ��41.1��
��6������Ũ����������NO��ϡ���������NO��������֤����װ�â���ˮ�������dz�ȥ��������е�NO2������ô�����NO��NaOH������������������ʢ��е�Һ��ΪŨ���ᣬ����Ϊϡ���ᣬװ�âٿ����ռ�NO��װ�â�Һ���Ϸ�������Ϊ��ɫ��װ�â�Һ���Ϸ�����������ɫ��Ϊ����ɫ��˵��Ũ����������Ա�ϡ����ǿ��
a��������������֪��װ�âڡ��ۡ�����ʢ�ŵ�ҩƷ������ϡ���ᡢˮ��Ũ���
b���ܹ�˵��ʵ���ѳɹ��������ǣ�װ�â�Һ���Ϸ�������Ϊ��ɫ��װ�â�Һ���Ϸ�����������ɫ��Ϊ����ɫ��
�ʴ�Ϊ��ϡ���ˮ��Ũ���װ����Һ���Ϸ�������Ϊ��ɫ��װ�ö�Һ���Ϸ�����������ɫ��Ϊ����ɫ��
���� �����ۺ���ǿ���漰ԭ�ӽṹ��Ԫ�ؼ��仯�������ʽ����ƽ�ͼ��㣬���ӵ�֪ʶ��Ҫ��ѧ�������ι̵Ļ���֪ʶ�����ù�ϵʽ���м���ļ��ܣ���Ŀ�Ѷ��еȣ�ע��ʵ����Ƶ�ԭ���ͷ�����

 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�| A�� | ԭ�Ӿ����У����ۼ��ļ���Խ���۷е�Խ�� | |
| B�� | ���Ӿ����У����Ӽ��������Խ�÷���Խ�ȶ� | |
| C�� | ���Ӿ����У����ۼ��ļ���Խ���۷е�Խ�� | |
| D�� | ԭ�Ӿ����У����ɾ������һ������ͬ��ԭ�� |
| A | Cl2��SO2��Ϻ������Ư��ֽ�� | Cl2��SO2���нϺõ�Ư������ |
| B | Һ̬�ƿ������˷�Ӧ�ѵĴ��Ƚ��� | �����ƾ���ǿ��ԭ�� |
| C | NH3��Һ�� | NH3������ˮ���Ӽ��γ���� |
| D | ���ǻ�����ȩ�е�������ǻ�����ȩ | ���ǻ�����ȩ���γɷ��Ӽ���������ǻ�����ȩ�γɷ�������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | VA=1.0 mol/��L•S�� | B�� | VB=0.6 mol/�� L•S�� | C�� | VC=1.6 mol/��L•S�� | D�� | VD=2.0 mol/��L•S�� |
| A�� | �ں�Fe3+��Cu2+��H+����Һ�м���п�ۣ�Cu 2+��Fe3+��H+ | |
| B�� | �ں�I-��SO32-��Br-����Һ�в���ͨ��������I-��Br-��SO32- | |
| C�� | �ں�AlO2-��SO32-��OH-����Һ����μ�������������Һ��OH-��AlO2-��SO32- | |
| D�� | �ں�Fe 3+��H+��NH4+ ����Һ�������ռ���Һ��Fe3+��NH4+��H+ |
| A�� | ǰ�ߴ� | B�� | ǰ��С | C�� | ��� | D�� | ����ȷ�� |
| A�� | CH3COOH+C2H5OH��CH3COOCH2CH3+H2O ȡ����Ӧ | |
| B�� | CH2=CH2+O2��CH3COOH �ӳɷ�Ӧ | |
| C�� | CH3CH2OH+CH3CH2OH��CH3CH2OCH2CH3+H2O ������Ӧ | |
| D�� | C6H6+HNO3��C6H5-NO2+H2O ������Ӧ |