��Ŀ����
�����£���20mL 0.2mol/L H2A��Һ�еμ�0.2 mol/L NaOH��Һ���й��������ʵ����仯��ͼ������I����H2A��II����HA-��III����A2-���������ʾ����NaOH�������������ͼʾ�жϣ�����˵����ȷ���ǣ� ��
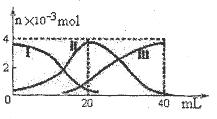

| A����V(NaOH)=20mLʱ����Һ������Ũ�ȴ�С��ϵ�� C(Na+)>c(HA-)>c(H+)>c(A2-)>c(OH-) |
| B��HA-�ĵ���̶�С��ˮ��̶� |
| C���μӹ����е���Һ������ʱ��V(NaOH)<20mL |
| D����V(NaOH)=40mLʱ����������Һ��ˮϡ�͵Ĺ�����pH���� |
A
��V(NaOH)=20mLʱ��H2A��NaOH�պ÷�Ӧ����4x10-3 mol NaHA������ͼ֪HA-
�����ʵ�����С��4x10-3 mol��֪c (Na+)>c(HA-)����A2-�����ʵ�����С��10-3 mol��
H2A��Ϊ0������HA-�������루c(HA-)>c(H+)������������ˮ�⣬��A2-��������H2A������
��HA-�ĵ���̶ȴ���ˮ��̶ȣ�HA-�ɵ����H+��A2-����ˮ����� H+�� OH-������
c(H+)>c(A2-)��c(H+)>c(OH-)����Һ�����ԣ�c(A2-)>c(OH-)��A2-�����ʵ�����С��10-3 mol��,
�μӹ����е���Һ������ʱ��V(NaOH)��20mL����A��ȷ��B����C������V(NaOH)=40mL
ʱ��H2A��NaOH�պ÷�Ӧ����4x10-3 mol Na2A,��ͼ֪A2-��ˮ�⣬��Һ�Գʼ��ԣ�������
��Һ��ˮϡ�͵Ĺ�������ٽ���A2-��ˮ�c(OH-)��С��pH��С��D����
�����ʵ�����С��4x10-3 mol��֪c (Na+)>c(HA-)����A2-�����ʵ�����С��10-3 mol��
H2A��Ϊ0������HA-�������루c(HA-)>c(H+)������������ˮ�⣬��A2-��������H2A������
��HA-�ĵ���̶ȴ���ˮ��̶ȣ�HA-�ɵ����H+��A2-����ˮ����� H+�� OH-������
c(H+)>c(A2-)��c(H+)>c(OH-)����Һ�����ԣ�c(A2-)>c(OH-)��A2-�����ʵ�����С��10-3 mol��,
�μӹ����е���Һ������ʱ��V(NaOH)��20mL����A��ȷ��B����C������V(NaOH)=40mL
ʱ��H2A��NaOH�պ÷�Ӧ����4x10-3 mol Na2A,��ͼ֪A2-��ˮ�⣬��Һ�Գʼ��ԣ�������
��Һ��ˮϡ�͵Ĺ�������ٽ���A2-��ˮ�c(OH-)��С��pH��С��D����

��ϰ��ϵ�д�
�����Ŀ
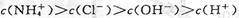
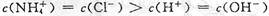
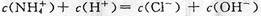
 CH3COO-+H+ ��H>0��
CH3COO-+H+ ��H>0�� ϡ��Һ�У�
ϡ��Һ�У� ��
�� ����֪
����֪ ����
���� ��ϡ������
��ϡ������ ��
�� =_________��
=_________�� ���
��� ��ijǿ����Һ��1���
��ijǿ����Һ��1��� ��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���
��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ��� ��ǿ���
��ǿ���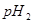 ֮��Ӧ����Ĺ�ϵ��_________��
֮��Ӧ����Ĺ�ϵ��_________��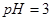 ��������Һ��
��������Һ�� ��
�� _________����>7��=7��<7����
_________����>7��=7��<7����