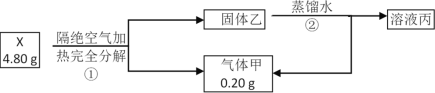
��Ŀ����
����Ŀ��ij��ҺX�п��ܺ������������е������֣�I����![]() ��
��![]() ��
��![]() ��Na����Mg2����Fe3+���������ӵ����ʵ���Ũ�Ⱦ���ͬ��Ϊ��ȷ������Һ����ɣ�ijͬѧȡ100mL������ҺX������������ʵ�飺
��Na����Mg2����Fe3+���������ӵ����ʵ���Ũ�Ⱦ���ͬ��Ϊ��ȷ������Һ����ɣ�ijͬѧȡ100mL������ҺX������������ʵ�飺
��1������ҺX�м���������Ba(OH)2��Һ���õ���ɫ������
��2������1���ķ�Ӧ���Һ���ˣ������������������У����������ܽ��Ҳ������塣
����˵����ȷ����
A.��2���в�����������ܺ���SO2
B.��ҺX��һ������![]() ��
��![]() ��Mg2+
��Mg2+
C.��ҺX��һ��������Fe3+�����ܴ���I��
D.��ȷ����Һ���Ƿ���Na+����Ҫ����ɫ��Ӧ����ȷ��
���𰸡�B
��������
��Һ��Ba(OH)2��Ӧ���ɰ�ɫ������Fe(OH)3�Ǻ��ɫ����һ������Fe3�����ң�1��������ɫ�������������ᷴӦ�����������ܽ��Ҳ������壬���ɫ����һ����BaSO4��һ�����ڣ�![]() ������
������![]() ��
��![]() �е�����һ�֣�����ΪCO2��SO2�е�����һ�֣���
�е�����һ�֣�����ΪCO2��SO2�е�����һ�֣���![]() ��Mg2����ٽ�ˮ�ⲻ�ܹ��棬�����������ӵ����ʵ���Ũ�Ⱦ���ͬ���ɵ���غ��֪��Һ�п϶���Na����Mg2������Һ��һ����
��Mg2����ٽ�ˮ�ⲻ�ܹ��棬�����������ӵ����ʵ���Ũ�Ⱦ���ͬ���ɵ���غ��֪��Һ�п϶���Na����Mg2������Һ��һ����![]() ��
��![]() ��Na����Mg2����������Fe3����
��Na����Mg2����������Fe3����![]() ��Cl����I����
��Cl����I����
A���ɷ�������ҺX��һ������![]() ��
��![]() ��Na����Mg2������2���в���������ΪCO2�������ܺ���SO2����A����
��Na����Mg2������2���в���������ΪCO2�������ܺ���SO2����A����
B���ɷ�����֪����ҺX��һ������![]() ��
��![]() ��Na����Mg2������B��ȷ��
��Na����Mg2������B��ȷ��
C����ҺX��һ��������Fe3����Ҳһ��������I������C����
D���ɷ�����֪����ҺX��һ������![]() ��
��![]() ��Na����Mg2����һ������Na+������Ҫ����ɫ��Ӧ����ȷ������D����
��Na����Mg2����һ������Na+������Ҫ����ɫ��Ӧ����ȷ������D����
��ѡB��

����Ŀ������(H3BO3)��Һ�д��ڣ�H3BO3(aq)��H2O(l)![]() [B(OH)4]��(aq)��H��(aq)������˵����ȷ����
[B(OH)4]��(aq)��H��(aq)������˵����ȷ����
��ѧʽ | ���볣��(298K) |
���� | K��5.7��10��10 |
̼�� | K1��4.4��10��7 K2��4.7��10��11 |
���� | K��1.75��10��5 |
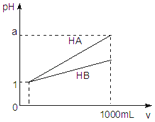
A.�����ʵ���Ũ�ȵ�̼������Һ�ʹ�������Һ�Ƚϣ�pH��ǰ�ߣ�����
B.�����ʵ���Ũ�ȵ�̼����Һ��������Һ�Ƚϣ�pH��ǰ�ߣ�����
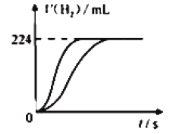
C.��һ��̼������Һ����������Һ��һ���ܹ۲쵽�����ݲ���
D.��һ�δ�����Һ����̼������Һ��һ���ܹ۲쵽�����ݲ���