��Ŀ����
ij�¶��£���0.2 mol C(s)��0.3 mol H2O(g)��Ͷ��2 L���ܱ������У�������ӦC(s)��H2O(g)  CO(g)��H2(g)��5 min�ﵽƽ����ܶ�������0.3 g��L��1���й�����˵����ȷ���ǣ� ��
CO(g)��H2(g)��5 min�ﵽƽ����ܶ�������0.3 g��L��1���й�����˵����ȷ���ǣ� ��
 CO(g)��H2(g)��5 min�ﵽƽ����ܶ�������0.3 g��L��1���й�����˵����ȷ���ǣ� ��
CO(g)��H2(g)��5 min�ﵽƽ����ܶ�������0.3 g��L��1���й�����˵����ȷ���ǣ� ��| A���ӷ�Ӧ��ʼ��ƽ������У���C����ʾ�÷�Ӧ��ƽ������Ϊ0.005 mol��L��1��min��1 |
| B����ƽ��ʱѹǿ��Ϊԭ����7/6 |
| C�����¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ0.012 5 |
| D���������¶Ⱥ�������䣬��ƽ����ϵ���ټ���0.2 mol C(s)��0.3 mol H2O(g)�����´ﵽƽ���H2O��ת���ʵ���16.7% |
B
C(s)�������� H2O(g)  CO(g)��H2(g)
CO(g)��H2(g)
��ʼ�� 0.2 mol 0.3 mol 0 0
ƽ�⡡ 0.2 mol��x 0.3 mol��x x x
��12x��0.3 g��L��1��2��x��0.05 mol
A�C�ǹ��壬��Ũ��Ϊ����������������ʾ��Ӧ���ʣ�����B�ѹǿ֮��Ϊ ��
�� ��
�� ��ȷ��C�K��
��ȷ��C�K�� ��0.005������D�ԭƽ��ʱ����(H2O)��
��0.005������D�ԭƽ��ʱ����(H2O)�� ��100%��16.7%���ټ���0.2 mol C(s)��0.3 mol H2O(g)ƽ�����ƣ�������Ӧ���������ķ�Ӧ��H2O(g)��ת���ʼ�С������
��100%��16.7%���ټ���0.2 mol C(s)��0.3 mol H2O(g)ƽ�����ƣ�������Ӧ���������ķ�Ӧ��H2O(g)��ת���ʼ�С������
 CO(g)��H2(g)
CO(g)��H2(g)��ʼ�� 0.2 mol 0.3 mol 0 0
ƽ�⡡ 0.2 mol��x 0.3 mol��x x x
��12x��0.3 g��L��1��2��x��0.05 mol
A�C�ǹ��壬��Ũ��Ϊ����������������ʾ��Ӧ���ʣ�����B�ѹǿ֮��Ϊ
 ��
�� ��
�� ��ȷ��C�K��
��ȷ��C�K�� ��0.005������D�ԭƽ��ʱ����(H2O)��
��0.005������D�ԭƽ��ʱ����(H2O)�� ��100%��16.7%���ټ���0.2 mol C(s)��0.3 mol H2O(g)ƽ�����ƣ�������Ӧ���������ķ�Ӧ��H2O(g)��ת���ʼ�С������
��100%��16.7%���ټ���0.2 mol C(s)��0.3 mol H2O(g)ƽ�����ƣ�������Ӧ���������ķ�Ӧ��H2O(g)��ת���ʼ�С������
��ϰ��ϵ�д�
�����Ŀ
 N2O4(��ɫ) ��H��0��Ӧ��NO2��N2O4�����ʵ����淴Ӧʱ��仯����������ͼ��������Ҫ������
N2O4(��ɫ) ��H��0��Ӧ��NO2��N2O4�����ʵ����淴Ӧʱ��仯����������ͼ��������Ҫ������

 N2��4CO2�ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ��Ӧ
N2��4CO2�ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ��Ӧ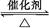 2SO3��һ��ʱ���,SO3��Ũ��������0.4 mol��L-1,�����ʱ������O2��ʾ�ķ�Ӧ����Ϊ0.04 mol��(L��s)-1,�����ʱ��Ϊ(����)
2SO3��һ��ʱ���,SO3��Ũ��������0.4 mol��L-1,�����ʱ������O2��ʾ�ķ�Ӧ����Ϊ0.04 mol��(L��s)-1,�����ʱ��Ϊ(����) CH3OH (g)+H2O(g) ��H=��49.0 kJ/mol
CH3OH (g)+H2O(g) ��H=��49.0 kJ/mol
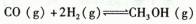


 2NO2(g)����H��0����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
2NO2(g)����H��0����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ� O2(g)
O2(g) SO3(g)����H����98 kJ/mol��ij�¶��£���һ���Ϊ2 L���ܱ������г���0.2 mol SO2��0.1 mol O2,5 min��ﵽƽ�⣬���ų�����11.76 kJ������˵����ȷ����(����)
SO3(g)����H����98 kJ/mol��ij�¶��£���һ���Ϊ2 L���ܱ������г���0.2 mol SO2��0.1 mol O2,5 min��ﵽƽ�⣬���ų�����11.76 kJ������˵����ȷ����(����)