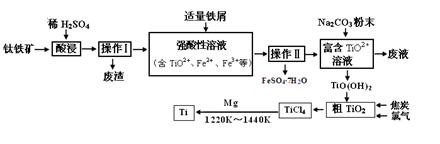
��Ŀ����
(1)ij����ÿ��Ҫ�յ�����1.6%����ú200 t,�ŷų���SO2��������Ⱦ����,������Ϊ��,����ЩSO2��������,��ô������ÿ��(��365 d��)������98%��Ũ������������;
(2)��Ҫ�����Ƽ��������,����Ӧ���Դ����������������,���Һ��������������Һ,��ƹ�������������Һ��Ũ�Ȼ���������(�������С�����䡱);
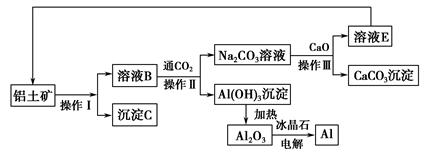
(3)��ҵ������ˮ�ࡢ����ʱ��Ҫ�õ���ԭ������������(����),����ѡԭ���Ʋ����Ļ�ѧ����ʽ���� ��;
(4)���������ֹ��̵���Ҫ�������� ;
(5)��������ˮ��ԭ����
(�����ӷ���ʽ��ʾ);������ʱӲ�ȵ�ˮ�г�ȥMg2+�ķ������� ��
�� (�û�ѧ����ʽ��ʾ)��
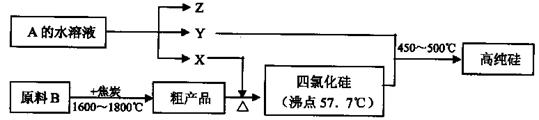
(2)��Ҫ�����Ƽ��������,����Ӧ���Դ����������������,���Һ��������������Һ,��ƹ�������������Һ��Ũ�Ȼ���������(�������С�����䡱);
(3)��ҵ������ˮ�ࡢ����ʱ��Ҫ�õ���ԭ������������(����),����ѡԭ���Ʋ����Ļ�ѧ����ʽ���� ��;
| A������ | B����ʯ�� | C��ʯ��ʯ | D����� |
(5)��������ˮ��ԭ����
(�����ӷ���ʽ��ʾ);������ʱӲ�ȵ�ˮ�г�ȥMg2+�ķ������� ��
�� (�û�ѧ����ʽ��ʾ)��
(1)3 650
(2)��������
(3)C��CaCO3+SiO2 CaSiO3+CO2��
CaSiO3+CO2��
(4)���������û�ԭ���������������л�ԭ����,�����������������������й����̼���������ʳ�ȥ
(5)Al3++3H2O Al(OH)3+3H+��Mg(HCO3)2
Al(OH)3+3H+��Mg(HCO3)2 MgCO3��+CO2��+H2O��MgCO3+H2O
MgCO3��+CO2��+H2O��MgCO3+H2O Mg(OH)2+CO2��
Mg(OH)2+CO2��
(2)��������
(3)C��CaCO3+SiO2
 CaSiO3+CO2��
CaSiO3+CO2��(4)���������û�ԭ���������������л�ԭ����,�����������������������й����̼���������ʳ�ȥ
(5)Al3++3H2O
 Al(OH)3+3H+��Mg(HCO3)2
Al(OH)3+3H+��Mg(HCO3)2 MgCO3��+CO2��+H2O��MgCO3+H2O
MgCO3��+CO2��+H2O��MgCO3+H2O Mg(OH)2+CO2��
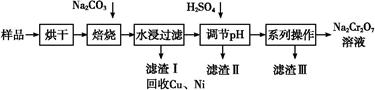
Mg(OH)2+CO2��(1)n(S)=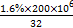 mol,n(H2SO4)=n(S)=105 mol,һ������m(H2SO4)=98��105 g,98%����������=1��107 g="10" t;(2)����Niʧȥ�����γ�������,�������õ��������ӵ����ʵ�����ͬ
mol,n(H2SO4)=n(S)=105 mol,һ������m(H2SO4)=98��105 g,98%����������=1��107 g="10" t;(2)����Niʧȥ�����γ�������,�������õ��������ӵ����ʵ�����ͬ
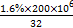 mol,n(H2SO4)=n(S)=105 mol,һ������m(H2SO4)=98��105 g,98%����������=1��107 g="10" t;(2)����Niʧȥ�����γ�������,�������õ��������ӵ����ʵ�����ͬ
mol,n(H2SO4)=n(S)=105 mol,һ������m(H2SO4)=98��105 g,98%����������=1��107 g="10" t;(2)����Niʧȥ�����γ�������,�������õ��������ӵ����ʵ�����ͬ
��ϰ��ϵ�д�
�����Ŀ
 ��������Na2CrO4+��������CO2+������������
��������Na2CrO4+��������CO2+������������  Cr2O72��+H2O
Cr2O72��+H2O