��Ŀ����
������ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H2��g��+CO��g��?CH3OH��g������H=-90.8kJ?mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g������H=-23.5kJ?mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g������H=-41.3kJ?mol-1
�ܷ�Ӧ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g���ġ�H=
����ͼ�ס����ǵ绯ѧʵ��װ�ã�
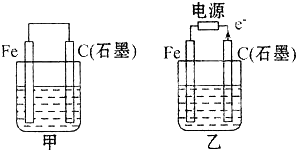
��1�����ס������ձ��о�ʢ�б���NaCl��Һ��
�ټ���ʯī���ϵĵ缫��Ӧʽ
�������ܷ�Ӧ�����ӷ���ʽΪ
�۽�ʪ��ĵ���-KI��ֽ�������ձ��Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ��
��2�����ס������ձ��о�ʢ��CuSO4��Һ��
�ټ��������ϵĵ缫��ӦʽΪ��
�������ʼʱ����ʢ��200mL pH=5��CuSO4��Һ��25�棩��һ��ʱ�����Һ��pH��Ϊ1����Ҫʹ��Һ�ָ������ǰ��״̬��������Һ�м���
��2H2��g��+CO��g��?CH3OH��g������H=-90.8kJ?mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g������H=-23.5kJ?mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g������H=-41.3kJ?mol-1
�ܷ�Ӧ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g���ġ�H=
-246.4
-246.4
kJ?mol-1������ͼ�ס����ǵ绯ѧʵ��װ�ã�

��1�����ס������ձ��о�ʢ�б���NaCl��Һ��
�ټ���ʯī���ϵĵ缫��Ӧʽ
2H2O+O2+4e-�T4OH-
2H2O+O2+4e-�T4OH-
�����ӵ��ƶ�����Ϊ��������ʯī
��������ʯī
���������ܷ�Ӧ�����ӷ���ʽΪ
2Cl-+2H2O
H2��+Cl2��+2OH-
| ||
2Cl-+2H2O
H2��+Cl2��+2OH-
��Cl-����
| ||
C
C
�缫����Fe��C�����۽�ʪ��ĵ���-KI��ֽ�������ձ��Ϸ���������ֽ�ȱ�������ɫ��������Ϊ������Cl2���������ɵ�I2������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ��
5Cl2+I2+6H2O�T10HCl+2HIO3
5Cl2+I2+6H2O�T10HCl+2HIO3
����2�����ס������ձ��о�ʢ��CuSO4��Һ��
�ټ��������ϵĵ缫��ӦʽΪ��
Fe-2e-�TFe2+
Fe-2e-�TFe2+
���������ʼʱ����ʢ��200mL pH=5��CuSO4��Һ��25�棩��һ��ʱ�����Һ��pH��Ϊ1����Ҫʹ��Һ�ָ������ǰ��״̬��������Һ�м���
CuO����CuCO3��
CuO����CuCO3��
����д���ʵĻ�ѧʽ��0.8����1.24��
0.8����1.24��
g��������I�����ø�˹���ɽ��
II����1���ټ�Ϊԭ���װ�ã�������������ʯī�������������������õ��ӣ����ӴӸ����ص�������������
����Ϊ���װ�ã��ɵ��������֪ʯīΪ�����������������ӷŵ磬�����������ӷŵ磬�������������ƶ���
��Cl2���������ɵ�I2��ClԪ�صĻ��ϼ۽��ͣ��ɵ����غ㼰Cl2��I2�����ʵ���֮��Ϊ5��1���жϷ�Ӧ��IԪ�صĻ��ϼۣ��Դ�����д��ѧ��Ӧ��
��2���ټ�Ϊԭ���װ�ã�������������ʧ���ӣ�
�ڸ��ݵ������ͭ�Ļ�ѧ��Ӧ��Ԫ���غ����жϼ��������ʹ��Һ�ָ������ǰ��״̬��������Һ��pH�ı仯����������ʵ�������
II����1���ټ�Ϊԭ���װ�ã�������������ʯī�������������������õ��ӣ����ӴӸ����ص�������������
����Ϊ���װ�ã��ɵ��������֪ʯīΪ�����������������ӷŵ磬�����������ӷŵ磬�������������ƶ���
��Cl2���������ɵ�I2��ClԪ�صĻ��ϼ۽��ͣ��ɵ����غ㼰Cl2��I2�����ʵ���֮��Ϊ5��1���жϷ�Ӧ��IԪ�صĻ��ϼۣ��Դ�����д��ѧ��Ӧ��
��2���ټ�Ϊԭ���װ�ã�������������ʧ���ӣ�
�ڸ��ݵ������ͭ�Ļ�ѧ��Ӧ��Ԫ���غ����жϼ��������ʹ��Һ�ָ������ǰ��״̬��������Һ��pH�ı仯����������ʵ�������
����⣺��2H2��g��+CO��g��?CH3OH��g������H=-90.8kJ?mol-1
��2CH3OH��g��?CH3OCH3��g��+H2O��g������H=-23.5kJ?mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g������H=-41.3kJ?mol-1
������ʽ�١�2+��+�ۿɵ�����Ӧ����ʽ�����H=-90.8kJ/mol��2-23.5kJ/mol-41.3kJ/mol=-246.4kJ/mol���ʴ�Ϊ��-246.4��
��1���ټ�Ϊԭ���װ�ã�ʯī���������õ��ӷ�����ԭ��Ӧ����ӦΪ2H2O+O2+4e-�T4OH-�����ӴӸ������ص�������ʯī��
�ʴ�Ϊ��2H2O+O2+4e-�T4OH-����������ʯī��
����Ϊ���װ�ã��ɵ��������֪ʯīΪ�����������������ӷŵ磬�����������ӷŵ磬
���ⷴӦΪ2Cl-+2H2O
H2��+Cl2��+2OH-��������������C�缫�ƶ���
�ʴ�Ϊ��2Cl-+2H2O
H2��+Cl2��+2OH-��C��
��Cl2���������ɵ�I2��ClԪ�صĻ��ϼ۽��ͣ��������ᣬ��Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1����IԪ�صĻ��ϼ�Ϊx����5��2��1=1��2��x�����x=+5�������ɵ��ᣬ
���Է����Ļ�ѧ��ӦΪ5Cl2+I2+6H2O�T10HCl+2HIO3��
�ʴ�Ϊ��5Cl2+I2+6H2O�T10HCl+2HIO3 ��
��2���ټ�Ϊԭ���װ�ã�����������������ӦΪFe-2e-�TFe2+���ʴ�Ϊ��Fe-2e-�TFe2+��
����2CuSO4+2H2O
2Cu+O2��+2H2SO4��Ҫʹ��Һ�ָ�ԭ״̬���ɼ���CuO����CuCO3����һ��ʱ�����Һ��pH��Ϊ1����c��H+��=0.1mol/L-10-5mol/L=0.1mol/L��
n��H+��=0.2L��0.1mol/L=0.02mol�����ɵ�ⷴӦ��֪������Cu�����ʵ���Ϊ0.01mol����Cuԭ���غ��֪��
m��CuO��=0.01mol��80g/mol=0.8g����m��CuCO3��=0.01mol��124g/mol=1.24g��
�ʴ�Ϊ��CuO����CuCO3����0.8����1.24����
��2CH3OH��g��?CH3OCH3��g��+H2O��g������H=-23.5kJ?mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g������H=-41.3kJ?mol-1
������ʽ�١�2+��+�ۿɵ�����Ӧ����ʽ�����H=-90.8kJ/mol��2-23.5kJ/mol-41.3kJ/mol=-246.4kJ/mol���ʴ�Ϊ��-246.4��
��1���ټ�Ϊԭ���װ�ã�ʯī���������õ��ӷ�����ԭ��Ӧ����ӦΪ2H2O+O2+4e-�T4OH-�����ӴӸ������ص�������ʯī��
�ʴ�Ϊ��2H2O+O2+4e-�T4OH-����������ʯī��
����Ϊ���װ�ã��ɵ��������֪ʯīΪ�����������������ӷŵ磬�����������ӷŵ磬
���ⷴӦΪ2Cl-+2H2O
| ||
�ʴ�Ϊ��2Cl-+2H2O
| ||
��Cl2���������ɵ�I2��ClԪ�صĻ��ϼ۽��ͣ��������ᣬ��Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1����IԪ�صĻ��ϼ�Ϊx����5��2��1=1��2��x�����x=+5�������ɵ��ᣬ
���Է����Ļ�ѧ��ӦΪ5Cl2+I2+6H2O�T10HCl+2HIO3��
�ʴ�Ϊ��5Cl2+I2+6H2O�T10HCl+2HIO3 ��
��2���ټ�Ϊԭ���װ�ã�����������������ӦΪFe-2e-�TFe2+���ʴ�Ϊ��Fe-2e-�TFe2+��
����2CuSO4+2H2O
| ||
n��H+��=0.2L��0.1mol/L=0.02mol�����ɵ�ⷴӦ��֪������Cu�����ʵ���Ϊ0.01mol����Cuԭ���غ��֪��
m��CuO��=0.01mol��80g/mol=0.8g����m��CuCO3��=0.01mol��124g/mol=1.24g��
�ʴ�Ϊ��CuO����CuCO3����0.8����1.24����
���������⿼��ԭ�������صĹ���ԭ������ȷ�缫��Ӧ�����õ����غ㡢Ԫ���غ�ȼ����ǽ����Ĺؼ����ѶȲ���

��ϰ��ϵ�д�
�����Ŀ


 ��1������ˮú���ϳɶ����ѣ�CH3OCH3�����Ȼ�ѧ����ʽΪ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g������H=-274KJ/mol���÷�Ӧ��һ�������µ��ܱ������дﵽƽ���Ϊͬʱ��߷�Ӧ���ʺͶ����ѵIJ��ʣ����Բ�ȡ�Ĵ�ʩ��
��1������ˮú���ϳɶ����ѣ�CH3OCH3�����Ȼ�ѧ����ʽΪ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g������H=-274KJ/mol���÷�Ӧ��һ�������µ��ܱ������дﵽƽ���Ϊͬʱ��߷�Ӧ���ʺͶ����ѵIJ��ʣ����Բ�ȡ�Ĵ�ʩ��