题目内容
【题目】人类的农业生产离不开氮肥,几乎所有的氮肥都以氨为原料,某化学兴趣小组利用如图装置制备氨气并探究相关性质。

(1)装置A 中,盛有浓氨水的仪器名称为_____。装置B 的作用是_____。
(2)连接好装置并检验装置的气密性后,装入药品,然后应先_____(填 I 或Ⅱ)。
Ⅰ.打开旋塞逐滴向圆底烧瓶中加入氨水 Ⅱ.加热装置C
(3)实验中观察到C 中 CuO 粉末变红,D 中无水硫酸铜变蓝,并收集到一种单质气体,则该反应相关化学方程式为_____,该反应证明氨气具有还原性;氨与氧气的在催化剂作用下的反应也体现了这一性质,该反应化学方程式为_____。
(4)该实验缺少尾气吸收装置,如图中能用来吸收尾气的装置是_____(填装置序号)。

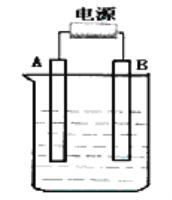
(5)实验室还可用如图所示装置制备氨气,化学反应方程式为_____。

(6)现将 1.92gCu 投入到一定量的浓HNO3 中,Cu 完全溶解,生成气体颜色越来越浅,共收集到标准状况下 672mL 的NOX 混合气体,将盛有此气体的容器倒扣在水槽中,通入标准状况下一定体积的O2,恰好使气体完全溶于水,则通入标准状况下的 O2 的体积为_____。
【答案】分液漏斗 干燥氨气 I 3CuO +2NH3 ![]() 3Cu + N2 + 3H2O 4NH3+5O2
3Cu + N2 + 3H2O 4NH3+5O2![]() 4NO + 6H2O II、III Ca(OH)2 + 2NH4Cl
4NO + 6H2O II、III Ca(OH)2 + 2NH4Cl![]() CaCl2 + 2NH3↑+2H2O 0.336L
CaCl2 + 2NH3↑+2H2O 0.336L
【解析】
浓氨水在碱石灰或生石灰的作用下挥发生成氨气,在B中用碱石灰干燥,在C中加热氨气与氧化铜发生氧化还原反应生成水、氮气和铜,D可用于检验是否生成水,最后氨气用水吸收,因氨气极易溶于水,注意防止倒吸。
(1)根据图中装置,装置A 中盛有浓氨水的仪器名称为分液漏斗,装置B是碱石灰,碱石灰不与氨气反应,起的作用是干燥氨气;故答案为:分液漏斗;干燥氨气。
(2)连接好装置并检验装置的气密性后,装入药品,然后应先打开旋塞逐滴向圆底烧瓶中加入氨水,再加热C处酒精灯;故答案为:I。
(3)实验中观察到C中CuO粉末变红,D中无水硫酸铜变蓝,并收集到一种单质气体,氨气中氮化合价升高,氧化铜中铜化合价降低,氢氧元素形成水,则该反应化学方程式为3CuO +2NH3 ![]() 3Cu + N2 + 3H2O,该反应证明氨气具有还原性;氨与氧气的在催化剂作用下的反应也体现了这一性质,该反应化学方程式为4NH3+5O2
3Cu + N2 + 3H2O,该反应证明氨气具有还原性;氨与氧气的在催化剂作用下的反应也体现了这一性质,该反应化学方程式为4NH3+5O2![]() 4NO + 6H2O;故答案为:3CuO +2NH3
4NO + 6H2O;故答案为:3CuO +2NH3 ![]() 3Cu + N2 + 3H2O;4NH3+5O2
3Cu + N2 + 3H2O;4NH3+5O2![]() 4NO + 6H2O。
4NO + 6H2O。
(4)该实验缺少尾气吸收装置,氨气极易溶于水,要注意防倒吸,II、III都能防倒吸,故答案为:II、III。
(5)实验室还用氯化铵和氢氧化钙加热反应生成氯化钙和氨气,其化学反应方程式为Ca(OH)2 + 2NH4Cl![]() CaCl2 + 2NH3↑+2H2O;故答案为:Ca(OH)2 + 2NH4Cl
CaCl2 + 2NH3↑+2H2O;故答案为:Ca(OH)2 + 2NH4Cl![]() CaCl2 + 2NH3↑+2H2O。
CaCl2 + 2NH3↑+2H2O。
(6)整个过程是铜失去电子的物质的量等于硝酸变为氮氧化物得到电子的物质的量,氮氧化物变为硝酸失去电子的物质的量与氧气得到电子的物质的量相等,即铜失去电子的物质的量等于氧气得到电子的物质的量,4n(O2)=2n(Cu),4n(O2)=2×![]() ,n(O2)=0.015mol,则通入标准状况下的 O2 的体积为V = nVm = 0.015mol× 22.4 L·mol1 =0.336L;故答案为:0.336L。
,n(O2)=0.015mol,则通入标准状况下的 O2 的体积为V = nVm = 0.015mol× 22.4 L·mol1 =0.336L;故答案为:0.336L。