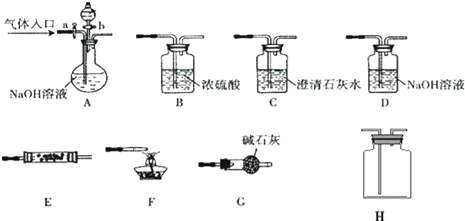
��Ŀ����
����Ŀ����֪�����Ȼ�ѧ����ʽ:��2H2(g)+O2(g)=2H2O(l) ��H=-571.6kJ��mol-1
��C3H8(g)+5O2(g)=3CO2(g) +4H2O(g) ��H=-2044.0kJ��mol-1
��1��������ȼ������__________
��2����֪��H2O(l)=H2O(g) ��H=+44.0kJ��mol-1��д������(C3H8)ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��__________
��3��ʵ����H2��C3H8�Ļ�����干3mol,��ȫȼ������Һ̬ˮʱ����2791.6kJ��������������H2��C3H8���������_____
��4�����º���������,����Է������·�Ӧ���䷴Ӧ���̺�������ϵ��ͼ��ʾ����֪2SO2(g)+O2(g)=2SO3(g) ��H=-196.6kJ��mol-1
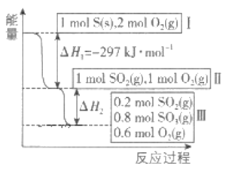
��д���ܱ�ʾ���ȼ���ȵ��Ȼ�ѧ����ʽ��__________
�ڡ�H2=__________kJ��mol-1
���𰸡�285.8 kJ��mol-1 C3H8(g)+5O2(g)=3CO2(g) +4H2O(l) ��H=-2220.0kJ��mol-1 3:1 S(s)+O2(g)=SO2(g) ��H=-297kJ��mol-1 -78.64kJ��mol-1
��������
(1) ������ȼ����ָ����1molH2��ȫȼ������ˮʱ�ų������������Ȼ�ѧ����ʽ��ȷ��������ȼ���ȣ�
(2)���Ȼ�ѧ����ʽ������֪H2O(l)=H2O(g) ��H=+44.0kJ��mol-1��Ͽɵó�����(C3H8)ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��
(3) ����������H2�����ʵ���Ϊxmol����ô571.6x+2220.0(3-x)=2791.6����û��������H2�����ʵ����ͱ�������ʵ������������ʵ���֮�ȵ������֮�ȵó����������H2��C3H8������ȣ�
(4) ��ȼ����ָ1mol��������ȫȼ�������ȶ���������ʱ�ų���������
�ڸ����Ȼ�ѧ����ʽ2SO2(g)+O2(g)=2SO3(g) ��H=-196.6kJ��mol-1���м��㡣
(1)���Ȼ�ѧ����ʽ�ٿ�֪������ȼ����Ϊ285.8 kJ��mol-1��
(2) ���Ȼ�ѧ����ʽ������֪H2O(l)=H2O(g) ��H=+44.0kJ��mol-1��ϣ��ó�����(C3H8)ȼ�����ɶ�����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ��C3H8(g)+5O2(g)=3CO2(g) +4H2O(l) ��H=-2220.0kJ��mol-1��
(3)����������H2�����ʵ���Ϊxmol����ô571.6x+2220.0(3-x)=2791.6�����x=2�������������H2�����ʵ���Ϊ2.3mol��C3H8�����ʵ���Ϊ0.7mol����˻��������H2��C3H8�������ԼΪ3:1��
(4) ��1molS(s)��ȫȼ������SO2(g)ʱ���ͷų�������Ϊȼ���ȣ�������ȼ���ȵ��Ȼ�ѧ����ʽΪ��S(s)+O2(g)=SO2(g) ��H=-297kJ��mol-1��
����ͼ��֪���뷴Ӧ��SO2�����ʵ���Ϊ1mol-0.2mol=0.8mol�������Ȼ�ѧ����ʽ2SO2(g)+O2(g)=2SO3(g) ��H=-196.6kJ��mol-1��֪��H2=![]() =0.4����H=0.4��(-196.6kJ��mol-1)=-78.64kJ��mol-1.
=0.4����H=0.4��(-196.6kJ��mol-1)=-78.64kJ��mol-1.

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��Ħ�����ڹ�ҵ������Ҫ����;����֪����һ�������ӣ�������������ɵľ��壬ijѧϰС�鰴����ʵ��ⶨĦ������Ʒ����ɡ��������£�
�ٳ�ȡ3.920gĦ������Ʒ����250mL��Һ��
��ȡ����������Һ������KSCN��Һ������������
����ȡ����������Һ���������Ũ����������Һ�����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ����������ͺ��ɫ������
�ܶ����ⶨ���£�
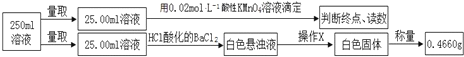
�ζ�ʵ������¼���£�
ʵ����� | ��һ�� | �ڶ��� | ������ |
���ĸ��������Һ���/mL | 10.32 | 10.02 | 9.98 |
���������գ�
(1)���������Ҫ�Ķ�������Ϊ________________ ��__________________��
(2)����ڵ�Ŀ����_____________________________________________________���������ɫ���������ӷ���ʽ_____________________________________��
(3)������в���XΪ_________________________________��������˳����д����
(4)����������Ը��������Һ�ܷ��õ�ľƾ���Һ���棬_______����ܡ����ܡ�������˵������__________________________________________________��
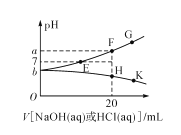
(5)��������ڵζ������У�����Һ���ã����ĸ��������Һ�������__________����ѡ� ƫ����ƫС�����䡱����
(6)ͨ������ʵ��ⶨ������ƶ�Ħ���λ�ѧʽΪ______________________________��