��Ŀ����
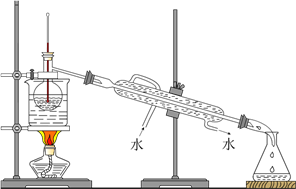
����Ŀ��K3Fe(A2B4)3��3H2O(M)���Ʊ������ͻ��������Ƶ���Ҫԭ�ϣ�Ҳ��һЩ�л���Ӧ������������й�ҵ������ֵ��A��B��Ϊ���������ڷǽ���Ԫ�ء�ij�о�С�齫�����Ļ�����M��һ�������¼��ȷֽ⣬��������������й���������ɽ���̽����
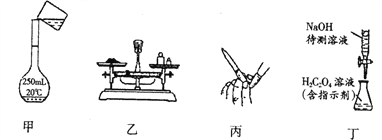
��1����ʵ�������������������ɼ����Һ�ˮ������ɡ��ס��Ҿ�ֻ��A��B����Ԫ�أ�����ʹ�����ʯ��ˮ�����ǣ��ҳ����ڹ�ҵ������
��д���ĵ���ʽ:_________��
��д����ҵ�����Ļ�ѧ��Ӧ����ʽ:_______________��
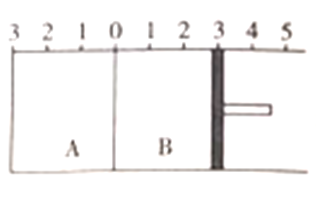
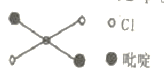
��2�����о�С��������Ϻ���֪����������в�����+3��������Ҳֻ��K2AB3��С��ͬѧ�Թ��������н�����ͼ��ʾ����������

������I�ľ������Ϊ____________��������������˵����������ж����е����ʵĻ�ѧʽΪ_____________��
��д��������KMnO4��Һ�����Ľ��������ӵ����ӽṹʾ��ͼ:_____________��������KMnO4��Һȥ�ζ�����Һʱ���յ���ɫ�仯Ϊ______________��
����֪������������KMnO4�Ļ�ԭ����ΪMn2+������ʵ�����ݷ��������������и����ʼ�����֮������ʵ���֮��Ϊ_____________��
��3��д������M���ȷֽ�Ļ�ѧ����ʽ:_______________��
���𰸡� ![]() 3CO+Fe2O3
3CO+Fe2O3![]() 2Fe+3CO2 ��ˮ�ܽ⣬���ˣ�δ��ˮ�ܽ⣬�����֣� Fe
2Fe+3CO2 ��ˮ�ܽ⣬���ˣ�δ��ˮ�ܽ⣬�����֣� Fe ![]() ��ɫ��Ϊdz��ɫ n(K2CO3)�Un(Fe)�Un(FeO)=3�U1�U1 2K3Fe(C2O4)3��3H2O
��ɫ��Ϊdz��ɫ n(K2CO3)�Un(Fe)�Un(FeO)=3�U1�U1 2K3Fe(C2O4)3��3H2O![]() 3K2CO3+Fe +FeO +5CO2��+4CO��+6H2O
3K2CO3+Fe +FeO +5CO2��+4CO��+6H2O
��������A��B��Ϊ���������ڷǽ���Ԫ�أ��ס��Ҿ�ֻ��A��B����Ԫ�أ�����ʹ�����ʯ��ˮ����ǣ��Ƴ�A��BΪ̼����Ԫ�ء��������⣬K3Fe(C2O4)3��3H2O �ֽ⣬ֻ��һ�������ɣ�K2CO3����ͬʱ��CO2��CO��������1���ټ��Ƕ�����̼������ʽΪ: ![]() ���ڹ�ҵ�����Ļ�ѧ��Ӧ����ʽΪ: 3CO+Fe2O3
���ڹ�ҵ�����Ļ�ѧ��Ӧ����ʽΪ: 3CO+Fe2O3![]() 2Fe+3CO2����2��������������ˮ�ܽ⣬���˺����ù���������ͭ��Һ��Ӧ��������������0.04g��˵���������ʴ��ڣ���ѧʽΪFe����������KMnO4��Һ��Ӧ�Ľ����������������ӵ����ӽṹʾ��ͼ:
2Fe+3CO2����2��������������ˮ�ܽ⣬���˺����ù���������ͭ��Һ��Ӧ��������������0.04g��˵���������ʴ��ڣ���ѧʽΪFe����������KMnO4��Һ��Ӧ�Ľ����������������ӵ����ӽṹʾ��ͼ: ![]() ��������KMnO4��Һȥ�ζ�����Һʱ���յ���ɫ�仯Ϊ��ɫ��Ϊdz��ɫ����
��������KMnO4��Һȥ�ζ�����Һʱ���յ���ɫ�仯Ϊ��ɫ��Ϊdz��ɫ����![]() �����������������ϡ���ᷴӦ��������KMnO4��Һ�ζ��������
�����������������ϡ���ᷴӦ��������KMnO4��Һ�ζ��������![]() ������0.01mol�����������ʣ�˵�������д���FeO��
������0.01mol�����������ʣ�˵�������д���FeO��![]() ����
����![]() ������n(K2CO3)�Un(Fe)�Un(FeO)=3�U1�U1����3�������������㣬����M���ȷֽ�Ļ�ѧ����ʽ: 2K3Fe(C2O4)3��3H2O
������n(K2CO3)�Un(Fe)�Un(FeO)=3�U1�U1����3�������������㣬����M���ȷֽ�Ļ�ѧ����ʽ: 2K3Fe(C2O4)3��3H2O![]() 3K2CO3+Fe +FeO +5CO2��+4CO��+6H2O��
3K2CO3+Fe +FeO +5CO2��+4CO��+6H2O��