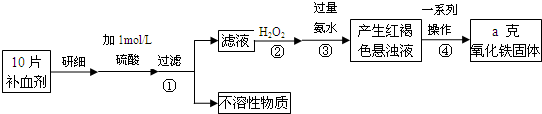
��Ŀ����
�������岻��ȱ�ٵ���Ԫ�أ����뺬���Ļ�����ɲ��������������ơ����г���һ�ֳ����IJ���ҩƷ���±���˵����IJ������ݡ�
|
[���]ÿƬ������������ [��Ӧ֢]����ȱ����ƶѪ֢��Ԥ���������á� [�����÷�]����Ԥ���� С������Ԥ���� [����]�ܹ⡢�ܷ⡢�ڸ��ﴦ���档 |
��1����ҩƷ��Fe2+�Ỻ�����������ҹ涨��ҩ����Fe2+�������ʣ��Ѿ�������Fe2+��������Fe2+�������ı�ֵ������10.00% �������ٷ��á�
��ʵ���ҿɲ���H2SO4�ữ��KMnO4��Һ���ԡ������ơ��е�Fe2+���еζ�(����ҩƷ�������ɷݲ���KMnO4��Ӧ)����д���÷�Ӧ�����ӷ���ʽ�� ��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ�IJ������������������ձ�����ͷ�ι��⣬���� ��
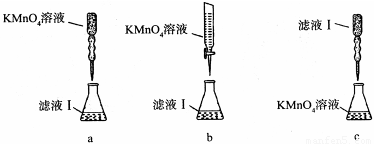
��ijͬѧ��������еζ���ʽ���гֲ�����ȥ������������� ��������ĸ��ţ�
��2��������������Ԫ����������Ϊ20.00%�ġ������ơ�10.00 g ������ȫ������ϡH2SO4�У����Ƴ�1000 ml��Һ��ȡ��20.00 ml����0.01000 mol•L-1��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00 ml ����ҩƷ��Fe2+��������Ϊ ��
��3����֪������Ϊ��Ԫ�л����ᣬ��23.6 g ���������Һ��4.0 mol•L-1 100.0 ml������������Һǡ����ȫ�к͡��˴Ź�����������ʾ�������������ͼ��ֻ���������շ塣д����������Һ������������Һ��ȫ�к͵Ļ�ѧ����ʽ(�л���д�ṹ��ʽ) ��
��1����5Fe2++MnO-4+8H+��5Fe3++Mn2++4H2O(2��) ��250ml����ƿ(2��)��b(2��)
��2��16.00% (3��)
��3��HOOC��CH2CH2��COOH��2NaOH��NaOOC��CH2CH2��COONa��2H2O (3��)
��������
�����������1����Fe2+���л�ԭ�ԣ������������ɳ�Fe3+�����������Һ����ǿ�����ԣ��������������ӣ�����MnO4-��Mn�Ļ��ϼ���+7�۽�Ϊ+2�ۣ��仯5��Fe2+��Fe��+2����Ϊ+3�ۣ��仯1�����ݻ��ϼ�����������Ⱥ������غ�÷�Ӧ�����ӷ���ʽΪ5Fe2++MnO-4+8H+��5Fe3++Mn2++4H2O��
��Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ�IJ������������������ձ�����ͷ�ι��⣬����250ml����ƿ��
��a�����������Һ����ǿ�����ԣ��ܸ�ʴ��ʽ�ζ��ܵ���Ƥ�ܣ���a����b�����������Һ����ǿ�����ԣ��ܸ�ʴ��ʽ�ζ��ܵ���Ƥ�ܣ�Ӧ����ʽ�ζ���ʢװ���ζ�ʱΪ���ڹ۲���ɫ�仯���ζ��յ���ɫ��dz�������ڹ۲죬Ӧ��������صε�����Һ�У���b��ȷ��c������������Һ�����ԣ�Ӧ�÷�����ʽ�ζ����У�ͬʱ�ζ��յ���ɫ�����dz����ڹ۲죬��c����ѡb��
��2����MnO4-��5Fe2+���ɵ�1000.00mL��Һ���е�Fe2+�����ʵ���n(Fe2+)��0.01 mol/L��12.00��10-3L��5�� ��0.03 mol��m(Fe2+)��0.03 mol��56 g/mol��1.68 g������Fe2+�������ʣ�
��0.03 mol��m(Fe2+)��0.03 mol��56 g/mol��1.68 g������Fe2+�������ʣ�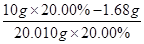 ��16.00%��
��16.00%��
��3�������������Ƶ����ʵ�����0.4mol����������ΪһԪ��ʱ��������Ӧ�Ĺ�ϵ������Է�������Ϊ23.6��0.4��59���������л������ԭ��������Ϊ��Ԫ��ʱ������Է�������Ϊ23.6��0.2��118���������������������λ�ò�ͬ����ԭ�ӣ�����������Ľṹ��ʽΪHOOC-CH2-CH2-COOH�������������Һ������������Һ��ȫ�к͵����ӷ���ʽΪHOOC��CH2CH2��COOH��2NaOH��NaOOC��CH2CH2��COONa��2H2O��
���㣺���������������Ӻ������ӵļ��飻������ԭ�ζ�����Ӧ���Լ�������ԭ��Ӧ�ļ��㣻���ӻ�ѧʽ��ȷ����

�������岻��ȱ�ٵ���Ԫ�أ����뺬���Ļ�����ɲ��������������ơ����г���һ�ֳ����IJ���ҩƷ���±���˵����IJ������ݡ���.��.��.Դ.��
| [���]ÿƬ������������ [��Ӧ֢]����ȱ����ƶѪ֢��Ԥ���������á� [�����÷�]����Ԥ���� С������Ԥ���� [����]�ܹ⡢�ܷ⡢�ڸ��ﴦ���档 |
(1) ��ҩƷ��Fe2+�Ỻ�����������ҹ涨��ҩ����Fe2+�������ʣ��Ѿ�������Fe2+��������Fe2+�������ı�ֵ������10.00% �������ٷ��á�
��ʵ���ҿɲ���H2SO4�ữ��KMnO4��Һ���ԡ������ơ��е�Fe2+���еζ�(����ҩƷ�������ɷݲ���KMnO4��Ӧ)����д���÷�Ӧ�����ӷ���ʽ��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ�IJ������������������ձ�����ͷ�ι��⣬���� ��
��ijͬѧ��������еζ���ʽ���гֲ�����ȥ������������� ��������ĸ��ţ�

(2) ������������Ԫ����������Ϊ20.00%�ġ������ơ�10.00 g ������ȫ������ϡH2SO4�У����Ƴ�1000 ml��Һ��ȡ��20.00 ml����0.01000 mol•L-1��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00 ml ����ҩƷ��Fe2+��������Ϊ ��
(3) ��֪������Ϊ��Ԫ�л����ᣬ��23.6 g ���������Һ��4.0 mol•L-1 100.0 ml������������Һǡ����ȫ�к͡��˴Ź�����������ʾ�������������ͼ��ֻ���������շ塣д����������Һ������������Һ��ȫ�к͵Ļ�ѧ����ʽ(�л���д�ṹ��ʽ)
��
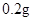 ��
��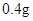 /�ա�
/�ա� ��
��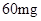 /�գ�������
/�գ�������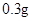 /��
/��