��Ŀ����
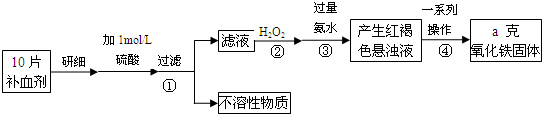
��ش��������⣺
��1���������H2O2��������ʹFe2+��ȫ��
��2����������õĺ��ɫ����Һ�г�H2O2����ˮ����Ҫ����
��3���������һϵ�д����IJ�������Ϊ
��4��ʵ�������Ѿ�ȷ��ȡ��Ũ��������100mL 1mol/L��������Һ��������ʱ�õ����������ձ�������������������������
��5����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص���������Ϊ
��6�����в����ᵼ�����ⶨ����Ԫ�غ���ƫ�ߵ���
A����������Ӱ�ˮ������ B�������ϴ�Ӳ���� C����������ղ���֣�
��2�����ɫ����Ϊ��������������������ܹ��ͼ���İ�ˮ��������泥�
��3�������������ȶ������ֽ⣬������Һ�еij������ֽ����Ҫ������;�����ˡ�ϴ�ӡ����ա���ȴ��������
��4����������һ�����ʵ���Ũ�ȵ���Һ����ѡ��ʹ�õ�������
��5������������������ag�����10Ƭ��Ѫ������Ԫ�ص����ʵ������������ټ����ÿƬ��Ѫ������Ԫ�ص�����������
��6��A����ˮ���㣬���ɵ������������٣��������������٣�
B��ϴ�Ӳ���֣��ᵼ������������ƫ��
C�����ղ���֣�������������ƫ����Ԫ������ƫ�ߣ�
��2�����ݷ�Ӧͼʾ�������������ܹ��백ˮ��Ӧ��������泥����ɫ�������������������ʴ�Ϊ����NH4��2SO4��Fe��OH��3��
��3���������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ������������Ȼ��������������������ȴ�������������������
�ʴ�Ϊ�����ˣ�
��4����ȷ����100mL 1mol/L��������Һ������ʱ��Ҫ����������ƽ��ҩ�ס��������ձ�����ͷ�ιܡ�100mL����ƿ�����Ի���Ҫ100mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��100mL����ƿ����ͷ�ιܣ�
��5��10Ƭ��Ѫ���������������ʵ���Ϊ��
| a |
| 160 |
| a |
| 160 |
| 7a |
| 10 |
| ||||
| b |
| 7a |
| b |
�ʴ�Ϊ��
| 7a |
| b |
��6��A����������Ӱ�ˮ�����㣬�����ӳ�������ȫ�����ɵ������������٣��������������٣��ⶨ���ƫ�ͣ���A����
B�������ϴ�Ӳ���֣��ᵼ��������������ƫ��õ���Ԫ����������ƫ��B��ȷ��
C����������ղ���֣��ᵼ�����������������ƫ����Ԫ����������ƫ��C��ȷ��
��ѡBC��

�������岻��ȱ�ٵ���Ԫ�أ����뺬���Ļ�����ɲ��������������ơ����г���һ�ֳ����IJ���ҩƷ���±���˵����IJ������ݡ���.��.��.Դ.��
| [���]ÿƬ������������ [��Ӧ֢]����ȱ����ƶѪ֢��Ԥ���������á� [�����÷�]����Ԥ���� С������Ԥ���� [����]�ܹ⡢�ܷ⡢�ڸ��ﴦ���档 |
(1) ��ҩƷ��Fe2+�Ỻ�����������ҹ涨��ҩ����Fe2+�������ʣ��Ѿ�������Fe2+��������Fe2+�������ı�ֵ������10.00% �������ٷ��á�
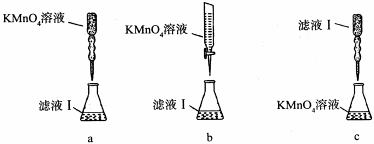
��ʵ���ҿɲ���H2SO4�ữ��KMnO4��Һ���ԡ������ơ��е�Fe2+���еζ�(����ҩƷ�������ɷݲ���KMnO4��Ӧ)����д���÷�Ӧ�����ӷ���ʽ��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ�IJ������������������ձ�����ͷ�ι��⣬���� ��
��ijͬѧ��������еζ���ʽ���гֲ�����ȥ������������� ��������ĸ��ţ�
(2) ������������Ԫ����������Ϊ20.00%�ġ������ơ�10.00 g ������ȫ������ϡH2SO4�У����Ƴ�1000 ml��Һ��ȡ��20.00 ml����0.01000 mol•L-1��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00 ml ����ҩƷ��Fe2+��������Ϊ ��
(3) ��֪������Ϊ��Ԫ�л����ᣬ��23.6 g ���������Һ��4.0 mol•L-1 100.0 ml������������Һǡ����ȫ�к͡��˴Ź�����������ʾ�������������ͼ��ֻ���������շ塣д����������Һ������������Һ��ȫ�к͵Ļ�ѧ����ʽ(�л���д�ṹ��ʽ)
��


