��Ŀ����
��4 mol A�����2 mol B������2L�������л�ϲ���һ�������·������·�Ӧ��2A(g)+B(g) 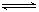 2C(g)����2s ����C��Ũ��Ϊ0.6mol?L-1�������м���˵����
2C(g)����2s ����C��Ũ��Ϊ0.6mol?L-1�������м���˵����
������ȷ���ǣ� ��
��������A��ʾ�ķ�Ӧ��ƽ������Ϊ0.3 mol?L-1?s-1
��������B ��ʾ�ķ�Ӧ��ƽ������Ϊ0.6 mol?L-1?s-1
�� 2sʱ����A��ת����Ϊ70%
�� 2sʱ����B��Ũ��Ϊ0.7 mol?L-1
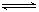 2C(g)����2s ����C��Ũ��Ϊ0.6mol?L-1�������м���˵����
2C(g)����2s ����C��Ũ��Ϊ0.6mol?L-1�������м���˵����������ȷ���ǣ� ��
��������A��ʾ�ķ�Ӧ��ƽ������Ϊ0.3 mol?L-1?s-1
��������B ��ʾ�ķ�Ӧ��ƽ������Ϊ0.6 mol?L-1?s-1
�� 2sʱ����A��ת����Ϊ70%
�� 2sʱ����B��Ũ��Ϊ0.7 mol?L-1
| A���٢� | B���٢ܡ����� | C���ڢۡ����� | D���ۢ� |
B
������淴Ӧ���йؼ��㣬һ���������ʽ����
2A(g)+B(g)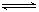 2C(g)
2C(g)
��ʼŨ�ȣ�mol/L�� 2 1 0
ת����Ũ�ȣ�mol/L�� 0.6 0.3 0.6
2min��Ũ�ȣ�mol/L�� 1.4 0.7 0.6
����������A��ʾ�ķ�Ӧ��ƽ������Ϊ0.6mol/L��2min��0.3mol/(L��min)
������B ��ʾ�ķ�Ӧ��ƽ������Ϊ0.3mol/L��2min��0.15mol/(L��min)
2sʱ����A��ת����Ϊ0.6��2��100����30��
2sʱ����B��Ũ��Ϊ0.7mol/L
���Դ�ѡB��
2A(g)+B(g)
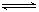 2C(g)
2C(g)��ʼŨ�ȣ�mol/L�� 2 1 0
ת����Ũ�ȣ�mol/L�� 0.6 0.3 0.6
2min��Ũ�ȣ�mol/L�� 1.4 0.7 0.6
����������A��ʾ�ķ�Ӧ��ƽ������Ϊ0.6mol/L��2min��0.3mol/(L��min)
������B ��ʾ�ķ�Ӧ��ƽ������Ϊ0.3mol/L��2min��0.15mol/(L��min)
2sʱ����A��ת����Ϊ0.6��2��100����30��
2sʱ����B��Ũ��Ϊ0.7mol/L
���Դ�ѡB��

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�
�����Ŀ
 N2��g��+CO2��g�� ��ij�о�С����ij�ܱյ������������������������䣬��������������Բ��ƣ��м���NO�������Ļ���̿�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����ұ���
N2��g��+CO2��g�� ��ij�о�С����ij�ܱյ������������������������䣬��������������Բ��ƣ��м���NO�������Ļ���̿�����£�T1�棩�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����ұ���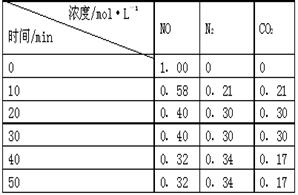
 PCl3(g)��Cl2(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±�������˵����ȷ����
PCl3(g)��Cl2(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±�������˵����ȷ���� 2C(g)+xD(g)����5min�ﵽƽ�⣬��ʱ����2 mol C�����D��ƽ����Ӧ����Ϊ0.15 mol/(L��min)����ƽ��ʱA�����ʵ���Ũ����____________��B��ת������__________��x��ֵ��___________��
2C(g)+xD(g)����5min�ﵽƽ�⣬��ʱ����2 mol C�����D��ƽ����Ӧ����Ϊ0.15 mol/(L��min)����ƽ��ʱA�����ʵ���Ũ����____________��B��ת������__________��x��ֵ��___________��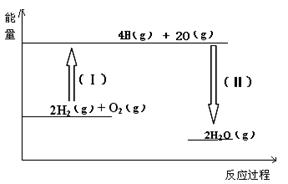
 2Z��g���ﵽƽ��ʱ����50%��Yת��ΪZ����X��ת����Ϊ25%������ʼʱ���������е�X��Y���ʵ���֮��Ϊ
2Z��g���ﵽƽ��ʱ����50%��Yת��ΪZ����X��ת����Ϊ25%������ʼʱ���������е�X��Y���ʵ���֮��Ϊ