��Ŀ����
��ˮD2O�����ӻ�Ϊ1.6��10-15��������PHһ���Ķ������涨pD=-lgc(D+),���¹���pD
��������ȷ����(���� )��
��������ȷ����(���� )��
| A��������Һ��PD=7 |
| B����1LD2O����Һ���ܽ�0.01mol NaOD������Һ�����Ϊ1L��������pD=12 |
| C����1L D2O���ܽ� 0.01mol DCl(����Һ�����Ϊ1L)������pD=2 |
| D����100mL 0.25mol��L-1��NaOD��ˮ��Һ�м���50mL 0.2mol��L-1DCl�ĵ���ˮ��Һ����pD=1 |
C
A��������ˮD2O�����ӻ�Ϊ1.0��10-14ʱ����Һ��PD=7��Ϊ������Һ��B��������ˮD2O�����ӻ�����Ϊ1.0��10-14��pD��12��C��ȷ��D������100mL 0.25mol��L-1��NaOD��ˮ��Һ�м���50mL 0.2mol��L-1DCl����Ӧ������0.015molNaOD����pDһ����Ϊ1��

��ϰ��ϵ�д�
�����Ŀ
 ��
��
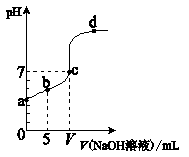
 H++OH�� ��H��0������������ȷ���ǣ� ��
H++OH�� ��H��0������������ȷ���ǣ� �� OH��)Ϊ
OH��)Ϊ