��Ŀ����
��10�֣���֪ 2 mol SO2 (g) ����Ϊ2 mol SO3 (g) �������仯��ͼ��ʾ��

��1��д��SO2 (g) ����ΪSO3 (g) ���Ȼ�ѧ����ʽ��
��2������SO2ʱ����ҵ�ϳ�����V2O5���������䷴Ӧ�����ɱ�ʾΪ
SO2 + V2O5 = SO3 + 2VO2�� 4VO2 + O2 = 2V2O5��
��ͼ�б�ʾ����V2O5�ķ�Ӧ����Ϊ ���a����b������
��3�������ݻ�Ϊ2 L���ܱ������г���2 mol SO2 (g)��1 mol O2 (g)����ͼ����ʾ����2 min�ﵽƽ�⣬��÷ų�����Ϊ178.2 kJ����

��2 min�� O2�Ļ�ѧ��Ӧ����v(O2)= ��
�����ٳ���1 mol O2���´ﵽƽ��ʱ��SO3ƽ��Ũ�ȵ�ȡֵ��Χ�ǣ� ��
��4������2 mol SO2 (g)��1 mol O2 (g)�����ݻ��ɱ���������У���ʼ���Ϊ2 L���ﵽƽ��ʱ�ų�����Q kJ����Q 178.2 kJ���>������=����<������

��1��д��SO2 (g) ����ΪSO3 (g) ���Ȼ�ѧ����ʽ��
��2������SO2ʱ����ҵ�ϳ�����V2O5���������䷴Ӧ�����ɱ�ʾΪ
SO2 + V2O5 = SO3 + 2VO2�� 4VO2 + O2 = 2V2O5��
��ͼ�б�ʾ����V2O5�ķ�Ӧ����Ϊ ���a����b������
��3�������ݻ�Ϊ2 L���ܱ������г���2 mol SO2 (g)��1 mol O2 (g)����ͼ����ʾ����2 min�ﵽƽ�⣬��÷ų�����Ϊ178.2 kJ����

��2 min�� O2�Ļ�ѧ��Ӧ����v(O2)= ��
�����ٳ���1 mol O2���´ﵽƽ��ʱ��SO3ƽ��Ũ�ȵ�ȡֵ��Χ�ǣ� ��
��4������2 mol SO2 (g)��1 mol O2 (g)�����ݻ��ɱ���������У���ʼ���Ϊ2 L���ﵽƽ��ʱ�ų�����Q kJ����Q 178.2 kJ���>������=����<������
��1��2SO2(g) + O2(g)  2SO3(g)����H=��198 kJ/mol ��2��b
2SO3(g)����H=��198 kJ/mol ��2��b
��3���� 0.225 mol��L��1��min��1 �� 0.9mol/L��c(SO3)<1 mol/L ��4����
 2SO3(g)����H=��198 kJ/mol ��2��b
2SO3(g)����H=��198 kJ/mol ��2��b��3���� 0.225 mol��L��1��min��1 �� 0.9mol/L��c(SO3)<1 mol/L ��4����
��1������ͼ���֪����Ӧ�Ƿ��ȷ�Ӧ�������Ȼ�ѧ����ʽΪ2SO2(g) + O2(g)  2SO3(g)����H=��198 kJ/mol��
2SO3(g)����H=��198 kJ/mol��
��2�������ܽ��ͷ�Ӧ�Ļ�ܣ������ܸı䷴Ӧ�ȣ����Դ���b��
��3���ٷų�����Ϊ178.2 kJ�������ĵ�������178.2��198��0.9mol�����������ķ�Ӧ������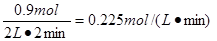 ��
��
���ڳ�������֮ǰ��������������1.8mol������Ӧ�ǿ��淴Ӧ���������۳������������������������ʵ������ܳ���2mol������Ũ�ȷ�Χ��0.9mol/L��c(SO3)<1 mol/L��
��4�����DZ��ֺ�ѹ�ģ�����Ϊ��Ӧ�������С�Ŀ��淴Ӧ���������з�Ӧ���ת����Ҫ���ڼ��еģ����ų�����������Q kJ��
 2SO3(g)����H=��198 kJ/mol��
2SO3(g)����H=��198 kJ/mol����2�������ܽ��ͷ�Ӧ�Ļ�ܣ������ܸı䷴Ӧ�ȣ����Դ���b��
��3���ٷų�����Ϊ178.2 kJ�������ĵ�������178.2��198��0.9mol�����������ķ�Ӧ������
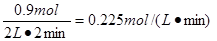 ��
�����ڳ�������֮ǰ��������������1.8mol������Ӧ�ǿ��淴Ӧ���������۳������������������������ʵ������ܳ���2mol������Ũ�ȷ�Χ��0.9mol/L��c(SO3)<1 mol/L��
��4�����DZ��ֺ�ѹ�ģ�����Ϊ��Ӧ�������С�Ŀ��淴Ӧ���������з�Ӧ���ת����Ҫ���ڼ��еģ����ų�����������Q kJ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �ų�942kJ�������ɴ��ж�����˵����ȷ���ǣ� ��
�ų�942kJ�������ɴ��ж�����˵����ȷ���ǣ� ��
 2SO3(g)����H =��Q1kJ/mol������ͬ�¶��£����ܱ�������ͨ��4molSO2��1molO2���ﵽƽ��ʱ�ų�����Q2 kJ�������й�ϵʽ��ȷ����
2SO3(g)����H =��Q1kJ/mol������ͬ�¶��£����ܱ�������ͨ��4molSO2��1molO2���ﵽƽ��ʱ�ų�����Q2 kJ�������й�ϵʽ��ȷ����