��Ŀ����
�����12�֣�
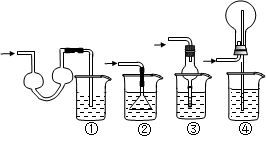
��ͼ��ij��ѧ��ȤС����ж����ѽ��ʵ�����̡���ע��CuO�ܽ���������CO2��H2O��G����װ��������أ�ʡ������̨�ȡ�������ͼ����װ�ú�����е�ʵ������У��ٸ�D��Gװ�ü��ȣ��ڼ������װ�õ������ԣ����ų�װ���еĿ����ȡ���

��1���������������Ⱥ�˳�������� ��д��ţ���
��2����Ҫ˵���ſ����ķ�����֤���������ž��ķ��� ��
��3��Bװ������������� ��
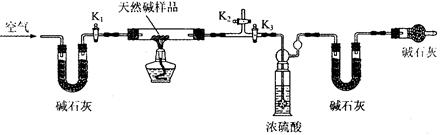
��4���ٶ����鰴C4H10��C2H6��C2H4��C4H10��CH4��C3H6�ķ�ʽ��ȫ�ѽ⣬����E��F��װ�õ��������ȷ�Ӧǰ������0.7g��Gװ�õ�����������1.76g��������ѽ�����У���������������ʵ���֮��Ϊ �����ٶ�����D��Gװ���е���������ȫ��Ӧ��
����Eװ���еĻ�����ٰ���������ʵ�飺

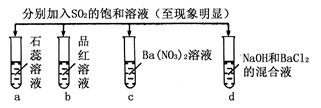
��5�����������͢�����Ʒֱ��ǣ��� �� ��Na2SO3��Һ�������ǣ������ӷ���ʽ��ʾ�� ��
��6��D�Ľṹ��ʽ�� ��
��ͼ��ij��ѧ��ȤС����ж����ѽ��ʵ�����̡���ע��CuO�ܽ���������CO2��H2O��G����װ��������أ�ʡ������̨�ȡ�������ͼ����װ�ú�����е�ʵ������У��ٸ�D��Gװ�ü��ȣ��ڼ������װ�õ������ԣ����ų�װ���еĿ����ȡ���

��1���������������Ⱥ�˳�������� ��д��ţ���
��2����Ҫ˵���ſ����ķ�����֤���������ž��ķ��� ��
��3��Bװ������������� ��
��4���ٶ����鰴C4H10��C2H6��C2H4��C4H10��CH4��C3H6�ķ�ʽ��ȫ�ѽ⣬����E��F��װ�õ��������ȷ�Ӧǰ������0.7g��Gװ�õ�����������1.76g��������ѽ�����У���������������ʵ���֮��Ϊ �����ٶ�����D��Gװ���е���������ȫ��Ӧ��
����Eװ���еĻ�����ٰ���������ʵ�飺

��5�����������͢�����Ʒֱ��ǣ��� �� ��Na2SO3��Һ�������ǣ������ӷ���ʽ��ʾ�� ��
��6��D�Ľṹ��ʽ�� ��
��1���ڢۢ�
��2������K�����ö��������ų���������С�Թ���Gװ�ú��ռ�һ�Թ����壬�ƽ������Ϸ�����ֻ�����������ı��������������������ž���2�֣�
��3�����ڹ۲춡����������ʣ��Ӷ����ƶ������������
��4��1�U1 ��2�֣�
��5����Һ ���� SO32����Br2��H2O��SO42����2Br����2H����2�֣�
��6��CH3CH(OH)COOH
��2������K�����ö��������ų���������С�Թ���Gװ�ú��ռ�һ�Թ����壬�ƽ������Ϸ�����ֻ�����������ı��������������������ž���2�֣�
��3�����ڹ۲춡����������ʣ��Ӷ����ƶ������������
��4��1�U1 ��2�֣�
��5����Һ ���� SO32����Br2��H2O��SO42����2Br����2H����2�֣�
��6��CH3CH(OH)COOH
��1��װ�����Ӻ��Ժ����ȼ��������ԣ�����˳���Ǣڢۢ١�
��2������װ�ÿ�֪�������ö������ž�װ���еĿ����������ǿ�ȼ�����壬ͨ�����鶡���������Ƿ��ž�������������K�����ö��������ų���������С�Թ���Gװ�ú��ռ�һ�Թ����壬�ƽ������Ϸ�����ֻ�����������ı��������������������ž���
��3��B����ˮ�����Կ���ͨ���۲����ݵ���������������٣��Ӷ����ƶ��������������
��4����������������ʵ����ֱ���x��y�����ϩ����ϩҲ�ֱ���x��y�����������֪ϩ����������0.7g������ͭ���ٵ���������ԭ�ӵ�����������������ϵ���ԭ����0.11mol������42x��28y��0.7��4x��7y��0.11�����x�Uy��1�U1��
��5�����������ܰ�ʣ�����ˮ��ԭ������ʽΪSO32����Br2��H2O��SO42����2Br����2H��������±����������ˮ�����Է�Һ���ɡ�����±�������ܣ����е�������ɡ�
��2������װ�ÿ�֪�������ö������ž�װ���еĿ����������ǿ�ȼ�����壬ͨ�����鶡���������Ƿ��ž�������������K�����ö��������ų���������С�Թ���Gװ�ú��ռ�һ�Թ����壬�ƽ������Ϸ�����ֻ�����������ı��������������������ž���
��3��B����ˮ�����Կ���ͨ���۲����ݵ���������������٣��Ӷ����ƶ��������������
��4����������������ʵ����ֱ���x��y�����ϩ����ϩҲ�ֱ���x��y�����������֪ϩ����������0.7g������ͭ���ٵ���������ԭ�ӵ�����������������ϵ���ԭ����0.11mol������42x��28y��0.7��4x��7y��0.11�����x�Uy��1�U1��
��5�����������ܰ�ʣ�����ˮ��ԭ������ʽΪSO32����Br2��H2O��SO42����2Br����2H��������±����������ˮ�����Է�Һ���ɡ�����±�������ܣ����е�������ɡ�

��ϰ��ϵ�д�
�����Ŀ


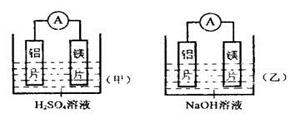
 xC(g) + D(g)����Ӧ�ﵽƽ��ʱC��Ũ��Ϊ1.2 mol/L��B��Ũ��Ϊ0.1 mol/L��
xC(g) + D(g)����Ӧ�ﵽƽ��ʱC��Ũ��Ϊ1.2 mol/L��B��Ũ��Ϊ0.1 mol/L��