��Ŀ����
15��ij��ɫ��Һ��ֻ���ܺ������������е������֣�K+��Mg2+��Fe3+��NH4+��Cl-��CO32-��SO42-����ÿ��ȡ100mL����ʵ�飺�ٵ�һ�ݼ���AgNO3��Һ�г���������
�ڵڶ��ݼ�������NaOH��Һ����������û�г������������ȣ��ռ�������1.12L����״����
�۵����ݼ�������BaCl2��Һ��ø������4.30g����������������ϴ�ӣ������ʣ��2.33g��
��������ʵ�飬�϶����ڵ�������NH4+��Cl-��CO32-��SO42-���϶������ڵ�������Mg2+��Fe3+������һ�����Ӳ���ȷ���Ƿ���ڣ�Ҫ�����������ӵ�ʵ�����������ȡ������Ʒ����ɫ��Ӧ������ɫ�ܲ����۲죬���������ɫ������K+��
���� �ɢ�ʵ���֪�����һ����Һ�м���AgNO3��Һ���а�ɫ������������ɫ����ΪAgCl��̼��������������
�ɢڿ�֪����ڶ�����Һ�м�������NaOH ��Һ����ȣ��ռ���1.12L����״�������壬����Ϊ�����������ʵ���Ϊ$\frac{1.12LL}{22.4L/mol}$=0.05mol��ԭ��Һ��һ����NH4+��
�ɢۿ�֪�����������Һ�м�������BaCl2��Һ���õ�����4.30g����������������ϴ�Ӻ�ʣ��2.33g����SO42-�����ʵ���Ϊ$\frac{2.33g}{233g/mol}$=0.01mol����CO32-�����ʵ���Ϊ$\frac{4.30g-2.33g}{197g/mol}$=0.01mol��������ӹ��桢����غ���
��� �⣺�ٵ�һ�ݼ���AgNO3��Һ�г�����������ɫ����ΪAgCl��̼��������������
����ڶ�����Һ�м�������NaOH ��Һ����ȣ��ռ���1.12L����״�������壬����Ϊ�����������ʵ���Ϊ$\frac{1.12LL}{22.4L/mol}$=0.05mol��ԭ��Һ��һ����0.05molNH4+��
�����������Һ�м�������BaCl2��Һ���õ�����4.30g����������������ϴ�Ӻ�ʣ��2.33g����SO42-�����ʵ���Ϊ$\frac{2.33g}{233g/mol}$=0.01mol����CO32-�����ʵ���Ϊ$\frac{4.30g-2.33g}{197g/mol}$=0.01mol��
��Һ��һ������NH4+��Cl-��CO32-��SO42-���������ӹ����֪ԭ��Һ��һ��������Fe3+��Mg2+��
�ɵ���غ㣬�ܸ���ɵ����ʵ���=0.01mol��2+0.01mol��2=0.04mol��������ɵ����ʵ���=0.05mol����֪һ������Cl-��
��������ӵķ���Ϊ��ȡ������Ʒ����ɫ��Ӧ������ɫ�ܲ����۲죬���������ɫ������K+��
�ʴ�Ϊ��NH4+��Cl-��CO32-��SO42-��Mg2+��Fe3+��ȡ������Ʒ����ɫ��Ӧ������ɫ�ܲ����۲죬���������ɫ������K+��
���� ���⿼�����ʵļ��鼰�ƶϣ�Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ����������뷴Ӧ�������ƶϵĹ�ϵΪ���Ĺؼ���ʵ����а�ɫ����Ϊ����ͻ�ƿڣ����ط�������㡢�ƶ������Ŀ��飬ע�����غ��Ӧ�ã�

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�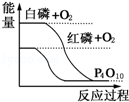 ��ͼ��ʾ�йط�Ӧ�ķ�Ӧ�����������仯�Ĺ�ϵ���ݴ��ж�����˵������ȷ���ǣ�������
��ͼ��ʾ�йط�Ӧ�ķ�Ӧ�����������仯�Ĺ�ϵ���ݴ��ж�����˵������ȷ���ǣ�������| A�� | �������İ�������׳��ȼ�գ����ų������� | |
| B�� | ���ױȰ����ȶ� | |
| C�� | ����ת��Ϊ���������ȷ�Ӧ | |
| D�� | ���ױȰ���������������Ӧ����P4O10 |
| A�� | ������ʯ��ʯ��������̼���� | |
| B�� | ���������Ȼ��ƣ������������� | |
| C�� | �������������ƣ������백ˮ | |
| D�� | ����ͭ���������ƣ�����ͭ���������� |
| A�� | 12.7 | B�� | 12.0 | C�� | 13.0 | D�� | 13.7 |
| A�� |  ��ȥCO�е�CO2 | B�� |  ��ȡʱ���Һ | ||
| C�� | 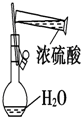 ����Ũ���� | D�� | 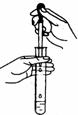 ���Թ��еμ�Һ�� |
| A�� | KNO3�Ǽ��Ρ������Σ�Ҳ������ | |
| B�� | ������ˮ��������ʯ�Ҷ��ǻ���� | |
| C�� | �л������������趼���л��߷��Ӳ��� | |
| D�� | BaSO4��Na3AlF6��NH4F����ǿ����� |
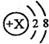 ����x����11���������ʾ��������������������ӣ���N��N2��2N���ַ����У�ֻ��ʾ�����壬������ʾ����������2N��
����x����11���������ʾ��������������������ӣ���N��N2��2N���ַ����У�ֻ��ʾ�����壬������ʾ����������2N�� ��
�� ��
��