��Ŀ����
��Ȳ���л��ϳɹ�ҵ��һ��ԭ�ϡ���ҵ������CaC2��ˮ��Ӧ������Ȳ��
��1��CaC2��ˮ��Ӧ������Ȳ�ķ�Ӧ����ʽΪ________.
��2���Ƚϵڶ�����Ԫ��C��N��O����Ԫ�صĵ�һ�����ܴӴ�С˳��Ϊ ______����Ԫ�ط��ű�ʾ������ԭ�ӽṹ�۵���Խ���______��
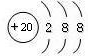
��3��CaC��C22����O22����Ϊ�ȵ����壬O22���ĵ���ʽ�ɱ�ʾΪ______��1molO22���к��е�������ĿΪ_______��
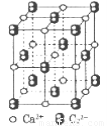
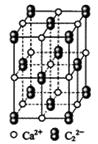
��4��CaC2����ľ����ṹ��NaCI��������ƣ���ͼ��ʾ������CaC2�����к��е���������C22���Ĵ��ڣ�ʹ������һ������������CaC2������1��Ca2����Χ���������C22����ĿΪ______��
��5������Ȳͨ��[Cu(NH3)2]Cl��Һ����Cu2C2����ɫ������Cu+��̬��������Ų�ʽΪ _______��
��6����Ȳ�������ᷴӦ�ɵñ�ϩ��(H2C=CH-C��N)����ϩ�������̼ԭ�ӹ���ӻ�������______�������д���ͬһֱ���ϵ�ԭ����Ŀ���Ϊ______��
��1��CaC2+2H2O=CH��CH��+Ca(OH)2��2)? N��O��C? C��N��Oԭ�Ӱ뾶���μ�С��ԭ�Ӻ˶������ӵ�������������ǿ��Ԫ�صĵ�һ��������������Oԭ�����������Ų�Ϊ2s22p4����Nԭ�����������Ų�Ϊ2s22p3��p�����Ų����ڰ����״̬�����ݺ��ع���������֪�������״̬���ȶ�������NԪ�صĵ�һ�����ܱ�O��3��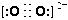 ? 2NA ��4��4? ��5��1s22s22p63s23p63d10? ��6��sp�ӻ� sp2�ӻ� 3
? 2NA ��4��4? ��5��1s22s22p63s23p63d10? ��6��sp�ӻ� sp2�ӻ� 3
��������
�����������1�����ݽ̲�֪ʶ��д������ʽΪCaC2+2H2O=CH��CH��+Ca(OH)2��
��2) �ڶ�����Ԫ��C��N��O����Ԫ�صĵ�һ�����ܴӴ�С˳��ΪN��O��C ����ΪC��N��Oԭ�Ӱ뾶���μ�С��ԭ�Ӻ˶������ӵ�������������ǿ��Ԫ�صĵ�һ��������������Oԭ�����������Ų�Ϊ2s22p4����Nԭ�����������Ų�Ϊ2s22p3��p�����Ų����ڰ����״̬�����ݺ��ع���������֪�������״̬���ȶ�������NԪ�صĵ�һ�����ܱ�O��
��3�����ݵȵ�����ԭ����֪��O22���ĵ���ʽ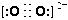 ����1��O22������2���м�����1 mol O22���У�����2NA���м���
����1��O22������2�������1 mol O22��������2NA�����
��4����CaC2����ľ����ṹ�ɿ�����CaC2������1��Ca2����Χ���������C22����ĿΪ4����
��5��Cu��29��Ԫ�أ���Cu����̬��������Ų�ʽΪ1s22s22p63s23p63d10��
��6���ڱ�ϩ�������̼ԭ�ӹ���ӻ����ʹ����ң���һ���ڶ���Cԭ�ӵ��ӻ�Ϊsp2�ӻ���������Cԭ�ӵ��ӻ���sp�ӻ��������д���ͬһֱ���ϵ�ԭ����Ŀ���Ϊ 3����?
���㣺������Ȳ�ķ��ӽṹ����ѧ���ʡ����Ԫ�صĵ����ܵıȽϼ��γɵĻ������ϩ��ȵĽṹ��֪ʶ��

 ��2010?���գ���Ȳ���л��ϳɹ�ҵ��һ��ԭ�ϣ���ҵ������CaC2��ˮ��Ӧ������Ȳ��
��2010?���գ���Ȳ���л��ϳɹ�ҵ��һ��ԭ�ϣ���ҵ������CaC2��ˮ��Ӧ������Ȳ��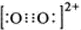
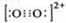
 ��ˮ��Ӧ������Ȳ��
��ˮ��Ӧ������Ȳ�� ��
�� ��Ϊ�ȵ����壬
��Ϊ�ȵ����壬 ����ĿΪ_
��
����ĿΪ_
�� ��Һ����
��Һ���� ,����ɫ������
,����ɫ������ ��̬��������Ų�ʽΪ
��
��̬��������Ų�ʽΪ
�� ��������ƣ�����ͼ��ʾ������
��������ƣ�����ͼ��ʾ������ ��Χ���������
�����������