��Ŀ����
����Ŀ����������һ�ִ��ж�ṹ��ϸС������ɵ������Ͻ𣬱��㷺�����л�����⻯��Ӧ�Ĵ������Ժ���������Ҫ�ɷ�ΪNiS��FeS��SiO2�ȣ�Ϊԭ���Ʊ��������Ĺ�����������ͼ��ʾ��

��1�����γ�Ni��CO��4�Ĺ����У�̼Ԫ�صĻ��ϼ�û�б仯����Ni��CO��4�е�Ni�Ļ��ϼ�Ϊ___________��
��2����֪�����������պ�����Ni2O3������ѹ��������ҺA�к���Ni2+��д���й���Ԫ�صļ�ѹ����Ļ�ѧ��Ӧ����ʽ______________________��
��3�������ҺA��ͨ��H2S���壬��Ӧ�����ӷ���ʽ��____________________��
��4��������������ʱ�ܷ�ͨ�������ΪCO��˵��ԭ��_____________________��
��5�����������Ŀ����ʹ�����Ͻ������ṹ���Ӷ���ǿ��������ǿ�����ԣ��˹���
�з�����Ӧ�����ӷ���ʽΪ___________________��������Ӧ���õ�NaOH��Һ��Ũ��Ҫ����NaOH��Һ��ϡʱ��������������Al��OH��3��������ֹ������Ӧ�ij������У����û�ѧ��Ӧԭ�����Խ��ͣ�________________________��
��6������ҺB���Ի��գ������������Ա�ѭ�����á�����ƼĻ������̣�
����ҺB��________________________����ʾ����CuOCu2+Cu��
���𰸡���1�� 0
��2��2Ni2O3+4H2SO4==4NiSO4+O2��+4H2O��2����
��3��H2S+2Fe3+==2Fe2++2H++S��2����
��4����������Ϊ��ȴʱ��CO����Ni��Ӧ����Ni��CO��4��2�֣�
��5��2Al��2OH����2H2O=2AlO2����3H2����2����
��ΪAl���ڼ�Һ����AlO2��ʱ��ˮ�д�������ƽ����AlO2��+2H2O![]() Al��OH��3+OH����OH��Ũ����С����AlO2����ˮ�����������С��������������Al��OH��3�ͻ��������������ֹ������Ӧ�ij������С���2�֣�
Al��OH��3+OH����OH��Ũ����С����AlO2����ˮ�����������С��������������Al��OH��3�ͻ��������������ֹ������Ӧ�ij������С���2�֣�
��6��Al��OH��3Al2O3![]() Al��
Al��
��������
�����������1�����γ�Ni��CO��4�Ĺ����У�̼Ԫ�صĻ��ϼ�û�б仯��+2�ۣ���Ni��CO��4�е�Ni�Ļ��ϼ�Ϊ0����2����֪�����������պ�����Ni2O3������ѹ��������ҺA�к���Ni2+����Ԫ�ػ��ϼ۽��ͣ�����ֻ������Ԫ�ػ��ϼ����ߣ���Ԫ�صļ�ѹ����Ļ�ѧ��Ӧ����ʽ2Ni2O3+4H2SO4==4NiSO4+O2��+4H2O����3�������ҺA�к��������ӣ�ͨ��H2S���壬��Ӧ�����ӷ���ʽ��H2S+2Fe3+==2Fe2++2H++S����4��������������ʱ����ͨ�������ΪCO����ȴʱ��CO����Ni��Ӧ����Ni��CO��4�����Բ�����ͨ�������ΪCO����5�����������������������������Һ������Ӧ�����ӷ���ʽΪ2Al��2OH����2H2O=2AlO2����3H2����������Ӧ���õ�NaOH��Һ��Ũ��Ҫ����NaOH��Һ��ϡʱ����ΪAl���ڼ�Һ����AlO2��ʱ��ˮ�д�������ƽ����AlO2��+2H2O![]() Al��OH��3+OH����OH��Ũ����С����AlO2����ˮ�����������С��������������Al��OH��3�ͻ��������������ֹ������Ӧ�ij������У���6������ҺB���Ի��գ������������Ա�ѭ�����á��������̣�����ҺB��Al��OH��3Al2O3
Al��OH��3+OH����OH��Ũ����С����AlO2����ˮ�����������С��������������Al��OH��3�ͻ��������������ֹ������Ӧ�ij������У���6������ҺB���Ի��գ������������Ա�ѭ�����á��������̣�����ҺB��Al��OH��3Al2O3![]() Al��
Al��

 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д� ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�����Ŀ�������е�Ԫ�����в������Ƚ�����Ƶ���
A�����ʳ��ˮ���ռ� |
B���ϳɰ��еĴ��ϳ� |
C�����������еĴ����� |
D������еİ���ˮ̼�ữ |
����ˮɹ�ε�±ˮ�л����Ȼ�þ����±ˮΪԭ������þ��һ�й�����������ͼ��ʾ��
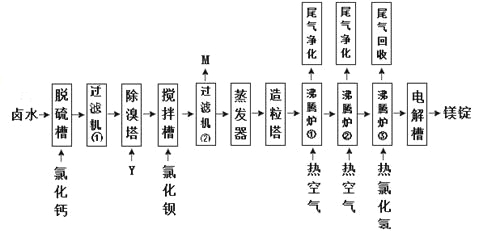
�ش��������⣺
��1������ۡ�����۾������ѳ�±ˮ�еģ������ӷ��ţ���M����Ҫ�ɷ��ǣ��ѧʽ����
��2������������Ҫ�����ӷ���ʽΪ��
��3������¯����������Ҫ�����ǡ�����¯��ͨ�����Ȼ������ҪĿ���ǡ�
��4�������������ĵ缫��Ӧ����ʽΪ��
��5����������������Ϊ���ò����ֱ�����ڱ����������еġ�