��Ŀ����
ij��Һ����Fe2+��Na+��Al3+��Ba2+��SO42-��NO3-��Cl-�е�4�����ӣ��������ӵ����ʵ�����Ϊ1mol���������Һ�м��������ϡ���ᣬ�����ݲ���������Һ������������䣨������ˮ�ĵ�������ӵ�ˮ�⣩������˵������ȷ����
| A���������Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù��������Ϊ72g |
| B���������Һ�м��������ϡ���ᣬ�����������������ܱ�ɺ���ɫ |
| C���������Һ�м��������ϡ�����KSCN��Һ����Һ��Ѫ��ɫ |
| D������Һ�������������ǣ�Fe2+��Na+��SO42-��NO3- |
A
���������������������֪�������Һ�м��������ϡ���ᣬ�����ݲ���������Һ������������䣬��һ������NO3- + 3Fe2+ + 4H+ = 3Fe3+ + NO�� + 2H2O��ԭ��Һһ������Fe2+��NO3-��������������䣬��ԭ��Һ�к���SO42-������Һ�к����������ӣ��������ӵ����ʵ�����Ϊ1mol���ɵ���غ��֪��ԭ��Һ��һ������Na+��A���������Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù���Ϊ��������������Ϊ0.5mol��160g/mol=80g������B�����ɵ�NO�ױ������ɺ���ɫ�Ķ�����������ȷ��C������Һ�м��ᷢ��������ԭ��Ӧ���������ӣ���KSCN��Һ����Һ��Ѫ��ɫ����ȷ��D���������ƶϿ�֪������Һ�������������ǣ�Fe2+��Na+��SO42-��NO3-����ȷ��
���㣺�������Ӽ���������ƶϡ�

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д��������ӷ���ʽ��д��ȷ����
| A����������NaOH��Һ����ͬŨ�ȵ�����Ca(HCO3)2��Һ��Ca2++2HCO3-+2OH-=CaCO3��+2H2O+CO32- |
| B����Ba(OH)2��Һ����μ���NH4HSO4��Һ���պó�����ȫBa2++OH-+H++ SO42-=BaSO4��+H2O |
C�����ð�˾ƥ�ֹ�������ˮ���ᣨ ����Ӧ���ɾ���ע��NaHCO3��Һ�� ����Ӧ���ɾ���ע��NaHCO3��Һ�� + 2 HCO3�� �� + 2 HCO3�� �� + 2 CO2�� + 2 H2O + 2 CO2�� + 2 H2O |
| D����FeI2��Һ�м�������ˮ 2Fe2++Cl2=2Fe3++2Cl- |
����ɫ����������Һ�У��ܴ���������������ǣ�������
| A��Fe2����Na����Cl�D��NO3�D | B��Ba2����Al3����Cl�D��NO3�D |
| C��K����Na����HCO3�D��Cl�D | D��AlO2-��K����OH�D��Cl�D |
ϡ���ǹ�ҵζ������Сƽͬ־˵�����ж���ʯ�ͣ�������ϡ������ϡ��Ԫ���棨Ce����Ҫ�����ڶ���ʯ�У��������ڿ������������䰵������ʱȼ�գ���ˮ�ܿ췴Ӧ����֪���泣���Ļ��ϼ�Ϊ+3��+4�������ԣ�Ce4+��Fe3+������˵����ȷ���� �� ��
| A���������CeO2��Ce������������� |
B�����������ȶ��ĺ���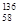 Ce�� Ce��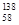 Ce�� Ce��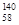 Ce�� Ce�� Ce�����ǻ�Ϊͬ�������� Ce�����ǻ�Ϊͬ�������� |
| C����Ce��SO4��2��Һ�ζ�����������Һ�������ӷ���ʽΪ��Ce4++Fe2+=Ce3++Fe3+ |
| D�����������ԣ�Ce4+��Fe3+����I-��ǿ��ԭ�ԣ����������������Ļ�ѧ����ʽ�ɱ�ʾΪ��2Ce+6HI=2CeI3+3H2��.���� |
���и�����������Һ���ܴ����������( )��
| A��Na����Al3����Cl����SO42- | B��Cu2����Cl����NO3-��OH- |
| C��Ca2����Na����CO32-��NO3- | D��H����SO42-��NO3-��OH�� |
�������ӷ���ʽ��д��ȷ����
A�����ҽ���Һ�м������ᡢ˫��ˮ��2I-��2H+��H2O2 I2��2H2O I2��2H2O |
B��CuƬ����FeCl3��Һ�У�Cu��Fe3+ Fe2++Cu2+ Fe2++Cu2+ |
C����Al2(SO4)3��Һ�м�������İ�ˮ�� Al3����4NH3��H2O  AlO2-��4NH4+��2H2O AlO2-��4NH4+��2H2O |
| D���� NaHSO4��Һ�еμ�Ba(OH)2��Һ�����ԣ� |
 BaSO4����H2O
BaSO4����H2O ����������ȷ����
| A���������ʶ��ܵ��磬�ǽ������ʶ����ܵ��� |
| B�����ӻ�����һ�������й��ۼ� |
| C��ֻ���й��ۼ�������һ���Ƿǵ���� |
| D��CaCl2������״̬�¿ɵ��磬����ǿ����� |
���и������ʵ�ϡ��Һ���Ӧ��������ǰ�ߵ�����ߣ����Ǻ��ߵ���ǰ�ߣ���Ӧ������ͬ����
| A��NaHSO4��Ba(OH)2 | B��AlCl3��NaOH | C��NaAlO2��H2SO4 | D��Na2CO3��H2SO4 |
���з��ӻ�������ָ���ķ�ɢϵ���ܴ��������һ����
| A��������Һ�У�ClO����Cl����K����Na+ |
| B�����³�ѹ�����壺O2��N2��Cl2��NH3 |
| C������AlO2-����Һ��NO3����HCO3����Na+��K+ |
| D�������������壺 H+��K+��S2����Br�� |