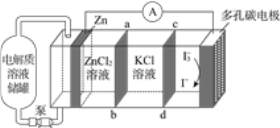
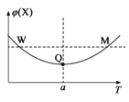
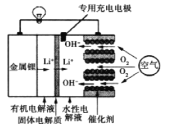
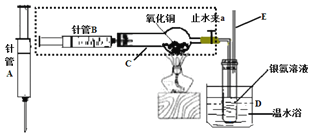
��Ŀ����
����Ŀ�������Ϻ�Ԫ����Ҫ��4He ��ʽ���ڣ������Ϻ�Ԫ����Ҫ��3He��ʽ���ڣ���֪һ��12Cԭ�ӵ�����Ϊa g��һ��3He ԭ�ӵ�����Ϊb g��һ��4He ԭ�ӵ�����Ϊc g �����У�
��3He��4He�Ļ�ѧ���ʻ�����ͬ ��3He��4He������ͬ�������� �ۺ�Ԫ�ص����ԭ������Ϊ![]() ��3He��4HeΪ��Ԫ�ص�ͬλ�أ�ͬ��ͬѹ���ܶ�֮��Ϊb:c��������ȷ���ǣ� ��
��3He��4HeΪ��Ԫ�ص�ͬλ�أ�ͬ��ͬѹ���ܶ�֮��Ϊb:c��������ȷ���ǣ� ��
A.�٢�B.�٢�C.�ڢ�D.�ۢ�
���𰸡�B
��������
��ͬ��Ԫ�ص�ԭ�ӻ�ѧ������ͬ��3He��4He��������ͬ����ͬ��Ԫ�ص�����ԭ�ӣ����Ի�ѧ������ͬ���ʢ���ȷ��
��4He��������Ϊ4-2=2��3He��������=3-2=1������������ͬ���ʢڴ���
��ijԪ�ص����ԭ�������ǰ�����Ԫ�صĸ��ֺ��ص����ԭ�������������İٷֺ��������ƽ��ֵ���ʢ۴���
��ͬ��ͬѹ���ܶ�֮��Ϊ����Ħ������֮�ȣ�3He ��Ħ������ΪbNA g/mol��4He ��Ħ������ΪcNA g/mol��ͬ��ͬѹ��3He��4He�ܶ�֮��Ϊb��c���ʢ���ȷ��
����������ΪB��

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д�
�����Ŀ