题目内容
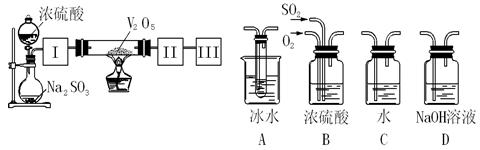
实验室中,用如下图所示装置及所给药品(图中部分夹持仪器已略去)探究工业制硫酸接触室中的反应,并测定此条件下二氧化硫的转化率。已知SO3熔点为16.8℃,假设气体进入装置时分别被完全吸收,且忽略装置内空气中的CO2。
(1)已知0.5molSO2被O2氧化成气态SO3,放出49.15 kJ热量,反应的热化学方程式为 。
(2)根据实验目的,请从上面右图中选择Ⅰ、Ⅱ、Ⅲ处合适的装置,将其序号填入空格中:装置Ⅰ ,装置Ⅱ ,装置Ⅲ 。
(3)开始进行实验时,首先应进行的操作是 。
(4)加热硬质玻璃管时,若不断升高温度,SO2的转化率会 (填“增大”、“不变”或“减小”)。
(5)停止通入SO2,熄灭酒精灯后,为使残留在装置中的SO2、SO3被充分吸收,操作方法是 。
(6)实验结束后,若装置Ⅱ增加的质量为b g ,装置Ⅲ增加的质量为a g,则此条件下二氧化硫的转化率是 (用含字母的代数表示)。

(1)已知0.5molSO2被O2氧化成气态SO3,放出49.15 kJ热量,反应的热化学方程式为 。
(2)根据实验目的,请从上面右图中选择Ⅰ、Ⅱ、Ⅲ处合适的装置,将其序号填入空格中:装置Ⅰ ,装置Ⅱ ,装置Ⅲ 。
(3)开始进行实验时,首先应进行的操作是 。
(4)加热硬质玻璃管时,若不断升高温度,SO2的转化率会 (填“增大”、“不变”或“减小”)。
(5)停止通入SO2,熄灭酒精灯后,为使残留在装置中的SO2、SO3被充分吸收,操作方法是 。
(6)实验结束后,若装置Ⅱ增加的质量为b g ,装置Ⅲ增加的质量为a g,则此条件下二氧化硫的转化率是 (用含字母的代数表示)。
(1)2SO2(g)+O2(g)  2SO3(g);△H=-196.6 kJ/mol (2分)
2SO3(g);△H=-196.6 kJ/mol (2分)
或 SO2(g)+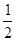 O2(g)
O2(g)  SO3(g);△H=-98.3 kJ/mol
SO3(g);△H=-98.3 kJ/mol
(2) B, A, D (3分)
(3)检查装置的气密性(1分)
(4)减小(1分)
(5)继续通入氧气一段时间(1分)
(6)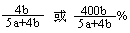 (2分)
(2分)
 2SO3(g);△H=-196.6 kJ/mol (2分)
2SO3(g);△H=-196.6 kJ/mol (2分)或 SO2(g)+
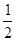 O2(g)
O2(g)  SO3(g);△H=-98.3 kJ/mol
SO3(g);△H=-98.3 kJ/mol (2) B, A, D (3分)
(3)检查装置的气密性(1分)
(4)减小(1分)
(5)继续通入氧气一段时间(1分)
(6)
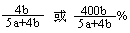 (2分)
(2分)试题分析:(1)ΔH与反应物的物质的量有关,所以热化学方程式中各物质的系数必须与ΔH相对应,如果系数加倍,则ΔH也要加倍。
(2)图中Ⅰ装置的作用一是干燥气体;二是使SO2和O2均匀混合,所以选B、Ⅱ装置的作用是冷却SO3,使其变成液体,所以选A,、Ⅲ装置的作用除去尾气SO2,所以选D (3分)
(3)一般说来,无论采用那种装置制取气体,在成套装置组装完毕装入反应物之前,必须检查装置的气密性,以确保实验的顺利进行。(1分)
(4)因为2SO2(g)+O2(g)
 2SO3(g)是放热反应,升高温度,向逆向移动,所以转化率减小。(1分)
2SO3(g)是放热反应,升高温度,向逆向移动,所以转化率减小。(1分)(5)继续通入氧气,可以使残留在装置中的SO2、SO3随着氧气流一起流动,最终全部被NaOH吸收。 (1分)
(6)实验结束后,若装置Ⅱ增加的质量为反应生成的SO3的质量,,装置Ⅲ增加的质量为末反应的SO2的质量,所以根据化学方程式,设反应的SO2质量为x;
2SO2(g)+O2(g)
 2SO3(g)
2SO3(g)128 160
x b
x=
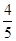 b
b所以二氧化硫的转化率为
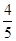 b/(a+
b/(a+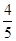 b)=
b)= (2分)
(2分)
练习册系列答案
 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案
相关题目
 HSO3-+OH-水解平衡的事实是________(填序号)。
HSO3-+OH-水解平衡的事实是________(填序号)。