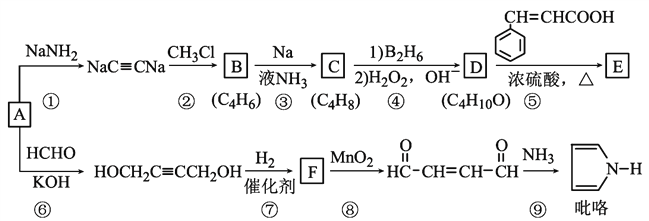
��Ŀ����
����Ŀ��ijNa2CO3��NaAlO2�Ļ����Һ����μ���1 mol/L�����ᣬ�����Һ��CO32-��HCO3-��AlO2-��Al3�������ʵ��������������Һ������仯��ϵ��ͼ��ʾ��������˵����ȷ����( )

A��a���߱�ʾ�����ӷ���ʽΪAlO2-��H����H2O==Al(OH)3��
B��ԭ�����Һ�е�CO32-��AlO2-�����ʵ���֮��Ϊ1��2
C��V1��V2��l��5
D��M��ʱ���ɵ�CO2Ϊ0��05 mol
���𰸡�A
��������
���������Na2CO3��NaAlO2�Ļ����Һ����μ���1molL-1��������ȣ�������ӦAlO2-+H++H2O�TAl(OH)3����a�߱�ʾAlO2-����ͼ��֪AlO2-��Ӧ��ϣ���������50mL�����ݷ���ʽ��֪n(AlO2-) = n(H+) = 0.05 L �� 1 mol/L = 0.05mol���ڶ��Σ�AlO2-��Ӧ��ϣ�������ӦCO32-+H+�THCO3-��b�߱�ʾCO32-��c�߱�ʾHCO3-����ͼ��֪CO32-��Ӧ��ϣ��ýμ�������100mL-50mL=50mL�����ݷ���ʽ��֪n(CO32-)=n(H+)=0.05L��1mol/L=0.05mol�������Σ�CO32-��Ӧ��ϣ�������ӦHCO3-+H+�TCO2��+H2O��d�߱�ʾHCO3-����ͼ��֪HCO3-��Ӧ��ϣ��ýμ�������150mL-100mL=50mL�����ݷ���ʽ��֪n(HCO3-)=n(H+)�����ĽΣ�������ӦAl(OH)3+3H+�TAl3++3H2O��e�߱�ʾAl3+����ͼ��֪Al(OH)3��Ӧ��ϣ����ݷ���ʽ��֪n(H+)=3n[Al(OH)3]=3��0.05mol=0.15mol���ýμ����������Ϊ![]() =0.15L=150mL��B��������������֪��ԭ�����Һ�е�CO32-��AlO-2�����ʵ���֮��Ϊ0.05mol��0.05mol=1��1����B����C��ԭ��Һ��n(CO32-)=0.05mol��V1ʱ��Һ��̼��������ӵ���̼�������Ϊ0.025ml���ɷ�ӦCO32-+H+�THCO3-��֪����Ҫ����Ϊ0.025mol����������Ϊ
=0.15L=150mL��B��������������֪��ԭ�����Һ�е�CO32-��AlO-2�����ʵ���֮��Ϊ0.05mol��0.05mol=1��1����B����C��ԭ��Һ��n(CO32-)=0.05mol��V1ʱ��Һ��̼��������ӵ���̼�������Ϊ0.025ml���ɷ�ӦCO32-+H+�THCO3-��֪����Ҫ����Ϊ0.025mol����������Ϊ![]() =0.025L=25mL����V1=50mL+25mL=75mL��������������֪��V2=150mL+150mL=300mL����V1��V2=75mL��300mL=l��4����C����D��������������֪M��ʱ��Һ��CO32-��ȫת��ΪHCO3-��û��CO2���ɣ���D����A��������������֪��a���߱�ʾ�����ӷ���ʽΪ��AlO-2+H++H2O=Al(OH)3������A��ȷ����ѡA��
=0.025L=25mL����V1=50mL+25mL=75mL��������������֪��V2=150mL+150mL=300mL����V1��V2=75mL��300mL=l��4����C����D��������������֪M��ʱ��Һ��CO32-��ȫת��ΪHCO3-��û��CO2���ɣ���D����A��������������֪��a���߱�ʾ�����ӷ���ʽΪ��AlO-2+H++H2O=Al(OH)3������A��ȷ����ѡA��

����Ŀ�����Ȼ���(TiCl4)����ȡ���칤ҵ���ϡ����ѺϽ����Ҫԭ�ϡ�ʵ������TiO2��CCl4Ϊԭ����ȡҺ̬TiCl4��װ����ͼ��ʾ�����ּг�װ��ʡ�ԣ���
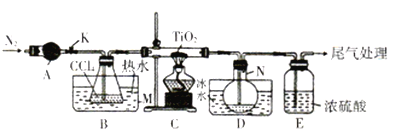
��֪���й����ʵ��������±���
���� | �۵�/�� | �е�/�� | ���� |
CCl4 | -23 | 76 | ��TiCl4���� |
TiCl4 | -25 | 136 | ����ʪ����������������550 ��ʱ�ܱ��������� |
��ش��������⣺
��1������A��������________������A��ʢװ���Լ���___________��
��2��CCl4�ĵ���ʽΪ__________��
��3��װ��C��Ӳ�ʲ����з�Ӧ�Ļ�ѧ����ʽΪ__________________��
��4��TiCl4����ʪ���������TiO2����Ӧ�Ļ�ѧ����ʽΪ________________��
��5������N���ռ�����������Ҫ��___________��д��ѧʽ�����Ӳ����з����TiCl4��ʵ�鷽����_____________��
��6��TiCl4������TiO2����̿�������ڼ����������Ƶã����������Ϊ2��1��CO��CO2������壬�÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ________�������ʵ�鷽����֤������������CO:_______________��
����Ŀ����̼��ѧ���о��ڹ�ҵ�����о�����Ҫ���塣
(1)��һ���¶Ⱥ�ѹǿ�£���֪��
��ѧ�� | C��H | C��O | O��H | C=O | O=O | C��C |
����(kJ��mol-1) | 414 | 326 | 464 | 728 | 498 | 332 |
��CH3CH2OH(g)+1/2O2(g)![]() CH3CHO(g)+H2O(g)��H1=____________��
CH3CHO(g)+H2O(g)��H1=____________��
����2CH3CHO(g)+O2(g)![]() 2CH3COOH(g)��Ӧ���Է����У�
2CH3COOH(g)��Ӧ���Է����У�
��CH3CH2OH(g)+O2(g)![]() CH3COOH(g)+H2O(g)��H2_____0(�>������<����=��)��
CH3COOH(g)+H2O(g)��H2_____0(�>������<����=��)��
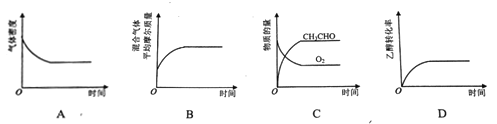
(2)��һ����CH3CH2OH��O2������¡���ѹ�ܱ������У�������Ӧ2CH3CH2OH(g)+O2(g)![]() 2CH3CHO(g)+2H2O(g)��ƽ��״̬������ͼ��������_________��
2CH3CHO(g)+2H2O(g)��ƽ��״̬������ͼ��������_________��
(3)��֪��25�棬Ka(CH3COOH)=1.75��10-5��Kb(NH3��H2O)= 1.75��10-5�� ![]() ��1.3��lgl.3��0.1
��1.3��lgl.3��0.1
��25�棬0.lmol��L-1CH3COOH ��Һ��pH =______����0.1 mol��L-1CH3COOH��Һ��0.1mol��L-1�İ�ˮ�������ϣ�������Һ������Ũ�ȴ�С��ϵΪ__________________��
��25�棬0.2 mol��L-1NH4Cl��Һ��NH4+ˮ�ⷴӦ��ƽ�ⳣ��Kh=_____ (����2λ��Ч����)��
��25�棬��0.1 mol��L-1��ˮ�м�������NH4Cl���壬NH3��H2O![]() NH4+ + OH-�ĵ���ƽ��_______������������桱���ߡ��������ƣ����ð�ˮ��ij����Σ������Լ�����Ʒ��ѡ)�����һ��ʵ��֤��NH3��H2O���������___________��
NH4+ + OH-�ĵ���ƽ��_______������������桱���ߡ��������ƣ����ð�ˮ��ij����Σ������Լ�����Ʒ��ѡ)�����һ��ʵ��֤��NH3��H2O���������___________��