��Ŀ����
��12�֣�����������Ԫ��X��Y��Z��W��ԭ������������������X��Y��Zλ�ڲ�ͬ���ڣ�Y���γɻ�������������Ԫ�أ�W2+��Neԭ�Ӿ�����ͬ�ĵ��Ӳ�ṹ��
��1����X��Y��ɵ�����������ijһȼ�ϵ�ص� ����Ӧ�
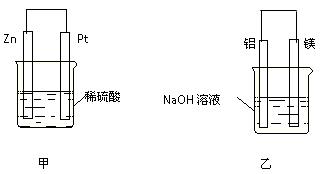
��2��Z ������ɵ�ij�ֻ��������ΪDZˮԱ�Ĺ��������û������к��еĻ�ѧ����
��3�����ȷ����У���W�ĵ��������ۡ�����ʳ����ɻ����A��ʹ��ʱ��ˮ����A�У������Ӻ˱����ˣ�������ת���Ƕȿ����ù����ǻ�ѧ��ת��Ϊ �ܣ�д��W��ˮ��Ӧ�Ļ�ѧ����ʽ��
��4��500��ʱ���ܱ������г���1mol ��L-1 CO2��3mol ��L-1 H2������Ӧ��
CO2(g)+3H2(g)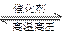 CH3OH(g) +H2O(g)
CH3OH(g) +H2O(g)
����й��������£�
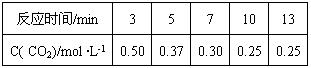
500��ʱ�÷�Ӧ��ƽ�ⳣ��K=" " ������һλС������ƽ��ʱCO2��ת����Ϊ ���¶����ߣ�Kֵ����������ӦΪ �ȷ�Ӧ��������š�����
��5����֪��298Kʱ��Ca(s) =Ca2��(g) +2e�� ; ��H="+" 1807kJ��mol��1
1/2O2(g)+2e-= O2- (g); ��H=+986kJ.mol-l
Ca2+(g) +O2-( g)=" CaO(s)" ; ��H="-" 3528. 5kJ.mol-l
298Kʱ�������ƺ�������Ӧ����CaO������Ȼ�ѧ����ʽΪ��
��1����X��Y��ɵ�����������ijһȼ�ϵ�ص� ����Ӧ�
��2��Z ������ɵ�ij�ֻ��������ΪDZˮԱ�Ĺ��������û������к��еĻ�ѧ����
��3�����ȷ����У���W�ĵ��������ۡ�����ʳ����ɻ����A��ʹ��ʱ��ˮ����A�У������Ӻ˱����ˣ�������ת���Ƕȿ����ù����ǻ�ѧ��ת��Ϊ �ܣ�д��W��ˮ��Ӧ�Ļ�ѧ����ʽ��
��4��500��ʱ���ܱ������г���1mol ��L-1 CO2��3mol ��L-1 H2������Ӧ��
CO2(g)+3H2(g)
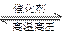 CH3OH(g) +H2O(g)
CH3OH(g) +H2O(g)����й��������£�

500��ʱ�÷�Ӧ��ƽ�ⳣ��K=" " ������һλС������ƽ��ʱCO2��ת����Ϊ ���¶����ߣ�Kֵ����������ӦΪ �ȷ�Ӧ��������š�����
��5����֪��298Kʱ��Ca(s) =Ca2��(g) +2e�� ; ��H="+" 1807kJ��mol��1
1/2O2(g)+2e-= O2- (g); ��H=+986kJ.mol-l
Ca2+(g) +O2-( g)=" CaO(s)" ; ��H="-" 3528. 5kJ.mol-l
298Kʱ�������ƺ�������Ӧ����CaO������Ȼ�ѧ����ʽΪ��
(1)����1�֣�
(2)���Ӽ����ۼ���1�֣�
(3)�ȣ�1�֣�Mg +2H2O Mg( OH)2+H2��2�֣�����д���۷֣�
Mg( OH)2+H2��2�֣�����д���۷֣�
(4)5.3��2�֣�75%��2�֣�����1�֣�
(5)Ca(s)��+ O2(g) �� CaO(s) ; ��H="-" 735. 5kJ��mol��1 (2�֣�
O2(g) �� CaO(s) ; ��H="-" 735. 5kJ��mol��1 (2�֣�
(2)���Ӽ����ۼ���1�֣�
(3)�ȣ�1�֣�Mg +2H2O
 Mg( OH)2+H2��2�֣�����д���۷֣�
Mg( OH)2+H2��2�֣�����д���۷֣�(4)5.3��2�֣�75%��2�֣�����1�֣�
(5)Ca(s)��+
 O2(g) �� CaO(s) ; ��H="-" 735. 5kJ��mol��1 (2�֣�
O2(g) �� CaO(s) ; ��H="-" 735. 5kJ��mol��1 (2�֣���

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д�
�����Ŀ
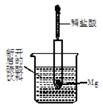



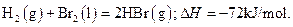 ����1mol Br2��l����Ҫ���յ�����Ϊ30kJ����������������±���
����1mol Br2��l����Ҫ���յ�����Ϊ30kJ����������������±���