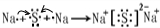��Ŀ����
A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ������������������A��DԪ��ͬ���壬B��CԪ��ͬ���ڣ���A��B��C��D�е�����Ԫ�ؿ��γ�ԭ�Ӹ�����Ϊ1:1�Ķ��ֻ�����ס��ҡ�������Ϊ���е����֣����ǵ�Ԫ��������±���ʾ��
| ������ | �� | �� | �� | �� |
| ���Ԫ�� | A��B | A��C | A��D | C��D |
ͨ��״���£�������Ϊ���壬�ܶ���С�ڿ�����������ΪҺ�壻�����ʺͶ�����Ϊ�����Ҷ�Ϊ���ӻ��������д���пհף�
��1�������ʵĽṹʽΪ �������ʵĵ���ʽΪ �����������������������ӵĸ�����Ϊ ��
��2������״����5.6L��������ȫȼ�������ȶ�������ʱ�ų�������Ϊ325kJ����д����ʾ������ȼ���ȵ��Ȼ�ѧ����ʽ ��
��3���о����������ʾ��������ԣ�����������ˮ�еĵ��뷽��ʽΪ ��
�� H��C��C��H , ��Na+ [:H] - ���� 1:2
�� C2H2(g)+�� O2(g)��2CO2(g)+H2O(l)����H����1300kJ/mol
�� H2O2 ![]() �� H+ +HO2- ��HO2-��
�� H+ +HO2- ��HO2-��![]() H+ +O22- (ֻҪд���˵�һ�����ɵ÷�)
H+ +O22- (ֻҪд���˵�һ�����ɵ÷�)

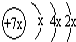 ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ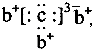 ���бȽ�����ȷ���ǣ�������
���бȽ�����ȷ���ǣ�������| A��ԭ�Ӱ뾶��a��c��d��b | B����ۺ����������c��d��a | C��ԭ��������a��d��b��c | D�����ʵ�������a��b��d��c |
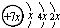 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺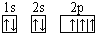

 Al��OH��3+OH-
Al��OH��3+OH-