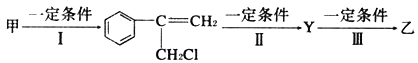��Ŀ����
����Ŀ��A��G�Ƕ���������Ԫ�أ�ԭ����������������Ԫ�صIJ�����Ϣ���±���ʾ��
A | B | C | D | E | F | |
ԭ�Ӱ뾶/nm | 0.077 | 0.075 | 0.074 | 0.099 | ||
��Ҫ ���ϼ� | +4 -4 | +5 -3 | -2 | +6 -2 | ||
���� | һ��ͬλ��ԭ�������� | �����л�����һ�����е�Ԫ�� | ���⻯�ﳣ��������� | ����������Ԫ����ԭ�Ӱ뾶��� |
�ش��������⣨����Ӧ��ѧ�����
��1��G�����ڱ��е�λ����___��F�ļ����ӽṹʾ��ͼ��___��
��2����A��C��G����Ԫ���γɵ�ԭ�Ӹ�����Ϊ4��1��1�Ļ�����ĵ���ʽΪ___���û����������Ļ�ѧ����___�����ѧ�����ͣ�
��3��D��E��F��G�����Ӱ뾶��С����˳��___��
��4���õ���ʽ��ʾBD2���γɹ���___��
��5��C��D��F�ļ��⻯���зе��ɸߵ��͵�˳����___��
��6��д��E2D2��A2D��Ӧ�Ļ�ѧ����ʽ___���÷�Ӧÿ����1molE2D2����ת����ĿΪ___��
���𰸡��������ڵڢ�A�� 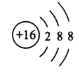
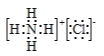 ���Ӽ��������ԣ����ۼ� Na+<O2-<Cl-<S2-
���Ӽ��������ԣ����ۼ� Na+<O2-<Cl-<S2- ![]() H2O>NH3>H2S 2Na2O2+2H2O=4NaOH+O2�� 2 NA
H2O>NH3>H2S 2Na2O2+2H2O=4NaOH+O2�� 2 NA
��������
Aͬλ��ԭ���������ӣ�˵��AΪ��Ԫ�أ������л���һ������B��˵��BΪ̼Ԫ�أ�C������⻯����������������CΪ��Ԫ�أ�D��ԭ�Ӱ뾶��C��С�ҳ������ϼ�Ϊ-2����DΪ��Ԫ�أ�EΪ����������Ԫ���а뾶���EΪ��Ԫ�أ�F�Ļ��ϼ����Ϊ+6��˵��FΪ��Ԫ�أ�������G��ԭ��������F��˵��G Ϊ��ԭ�ӡ�
��1��GΪ��Ԫ�أ������ڱ��е�λ���ǵ������ڵڢ�A�壬FΪ��Ԫ�أ������ӽṹʾ��ͼ�� ��
��
��2����A��C��G����Ԫ���γɵ�ԭ�Ӹ�����Ϊ4��1��1�Ļ�����Ϊ�Ȼ�泥�����ʽΪ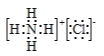 ���û����������Ļ�ѧ�������Ӽ��ͣ����ԣ����ۼ���
���û����������Ļ�ѧ�������Ӽ��ͣ����ԣ����ۼ���
��3������ԭ�Ӻ�Լ�����������ӵİ뾶С�������ӣ��˵����Խ�뾶ԽС��D��E��F��G�����Ӱ뾶��С����˳��Na+<O2-<Cl-<S2-��
��4���õ���ʽ��ʾBD2��������̼���γɹ���![]() ��
��
��5��C��D��F�ļ��⻯��������������е����һ���⻯���Է�����������,���Ӽ�����������,�е����ߡ�ˮ�Ͱ������Ӽ����������е�������⣬�е��ɸߵ��͵�˳����H2O>NH3>H2S��
��6��E2D2��A2D��Ӧ�Ļ�ѧ����ʽ2Na2O2+2H2O=4NaOH+O2��������������ԭ��Ӧԭ�����÷�Ӧÿ����1molE2D2����ת����ĿΪ2 NA��