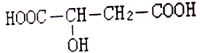��Ŀ����
����Ŀ�����������л��ϳ��е���Ҫԭ�ϡ�ʵ�����Ʊ�������(C2H5Br���е�38.4��)��װ����ͼ��ʾ����ʵ�鲽��Ϊ���ټ��װ�õ������ԣ���װ��ͼ��ʾ��U�ιܺʹ��ձ��м����ˮ����������a�м���10mL 95%�Ҵ���28mL 92%Ũ���ᣬȻ����������廯�ƺͼ������Ƭ������45~50�����2h,ʹ���ַ�Ӧ���ش��������⣺
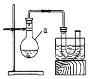
(1)����a��������________��
(2)�ڴ��ձ��м����ˮ��Ŀ����________��
(3)�������Ƭ��������________��
(4)Ϊ�˸��õĿ����¶ȣ�ѡ���õļ��ȷ�ʽΪ________��
(5)��Ӧʱ���¶ȹ��ߣ��ɿ����к���ɫ������������������ʽΪ________�����ɵ���ɫ�̼�����ζ����ķ���ʽΪ________��
(6)U�ι��ڿɹ۲쵽��������_____________��
(7)��Ӧ������U�ι��ڴ��Ƶ�C2H5Br���ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е����ʣ�����ѡ�������Լ��е�________(�����)��
A��Na2SO3��Һ B��H2O C��NaOH��Һ D����
(8)��������������Ԫ�ص�ʵ�鲽���ǣ�ȡ�������������Թ��У�����NaOH��Һ���������һ��ʱ�䣬��ȴ��________��
���𰸡� Բ����ƿ ʹC2H5Br������Һ̬ ��ֹ���� ˮԡ���� Br2 SO2 ����״Һ������ A ����ϡHNO3�ữ���ټ���AgNO3��Һ���۲��Ƿ����ɻ�ɫ����
��������92%Ũ����������廯�Ʒ�Ӧ�����廯�⣬�ٺ��Ҵ���Ӧ���������飬�������Ƭ�������Ƿ�ֹ���У�Ϊ�˸��õĿ����¶ȣ�ѡ���õļ��ȷ�ʽΪˮԡ���ȣ���Ӧʱ���¶ȹ��ߣ�Ũ���Ὣ�������������嵥���ܽ����л�������ʾ�ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е������嵥�ʣ�������������������֮������Ӧ���ɵ���Һ�������黥�����ܣ������������к�����Ԫ��һ��Ҫ��֮ת��Ϊ�����ӣ����Բ���±������ˮ�ⷽ�����������������Ƽ��ɣ��������ӿ��Ժ������ӷ�Ӧ���ɵ���ɫ����������ij����廯�������飬���Լ��������ữ����������
(1)����a��������Բ����ƿ��(2)������ķе�ϵͣ��ڴ��ձ��м����ˮ��Ŀ����ʹC2H5Br������Һ̬��(3)Ϊ��ʵ�鰲ȫ���������Ƭ�������Ƿ�ֹ���С�(4)ˮԡ������ʹ���Ⱦ��ȣ�Ϊ�˸��õĿ����¶ȣ�ѡ���õļ��ȷ�ʽΪˮԡ���ȡ�(5)��Ӧʱ���¶ȹ��ߣ�Ũ���Ὣ�������������ɿ����к���ɫ������������������ʽΪBr2�����ɵ���ɫ�̼�����ζ����ķ���ʽΪSO2 ��(6)U�ι����ռ���������(C2H5Br���е�38.4��)���ɹ۲쵽������������״Һ�����ɡ�(7)��Ӧ������U�ι��ڴ��Ƶ�C2H5Br���ػ�ɫ�����л����嵥�ʡ��嵥���ܽ����л�������ʾ�ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е������嵥�ʣ�������������������֮������Ӧ���ɵ���Һ�������黥�����ܣ��ʴ�ΪC��NaOH��Һ��(8)��������������Ԫ�ص�ʵ�鲽���ǣ�ȡ�������������Թ��У�����NaOH��Һ���������һ��ʱ�䣬��ȴ������ϡHNO3�ữ���ټ���AgNO3��Һ���۲��Ƿ����ɻ�ɫ������