��Ŀ����
ʵ������һƿ�����Ȼ��Ƶ��������ƹ����Լ������ⶨ�������Ƶ���������ԼΪ82%��Ϊ����֤�䴿�ȣ���Ũ��Ϊ0.2 mol��L��1��������еζ�������������⣺
(1)��ȡ5.0 g���������ƹ�����Ʒ�����500 mL��Һ���á�
(2)��������װ��25.00 mL��________�ζ����У�����Һ��λ���ڡ�0���̶����£�����¼�¿̶ȡ�
(3)ȡ20.00 mL����Һ������ʵ�����ʹ�õ���Ҫ������________���÷�̪��ָʾ��ʱ���ζ�����Һ��ɫ��________ɫ�պñ��________ɫΪֹ��
(4)�ζ����յ����������ȥ20.00 mL�������������Ƶ���������Ϊ________��
(5)�Է��������ζ���������������Щʵ���������________(�����)��
| A��ת�ƴ���Һ������ƿʱ��δϴ���ձ� |
| B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���� |
| C���ζ�ʱ��Ӧ��ҡ��̫���ң�������Һ�彦�� |
| D���ζ����յ�ʱ���ζ��ܼ����������� |
(2)��ʽ��(3)��ʽ�ζ��ܡ���ƿ���졡��
(4)80%��(5)ACE
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д����й���0.1 mol��L��1CH3COONa��Һ��˵����ȷ���� �� ��
| A����������NaOH���壬c(CH3COO��)���� |
| B����������FeCl3���壬c(CH3COO��)���� |
| C��ϡ����Һ����Һ��pH���� |
| D�������������ᣬ�õ������Ի����Һ��c(Na��)��c(CH3COO��) |
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
| ʵ���� | HA | NaOH | �����Һ��pH |
| �� | [HA]��0.2 mol��L��1 | [NaOH]��0.2 mol��L��1 | pH��a |
| �� | [HA]��c1 mol��L��1 | [NaOH]��0.2 mol��L��1 | pH��7 |
| �� | [HA]��0.1 mol��L��1 | [NaOH]��0.1 mol��L��1 | pH��9 |
| �� | pH��2 | pH��12 | pH��b |
��ش�
(1)�������������ʵ���������Ӽ�����������������a(�����Һ��pH)��˵��HA��ǿ�ỹ�����________________________________________________
(2)c1________(�����������������)0.2 mol��L��1������ʵ����HA��NaOH��Һ���ǰ��HA��Һ��[A��]��NaOH��Һ��[Na��]�Ĺ�ϵ��________(������ѡ����ѡ�����)��
A��ǰ�ߴ� B�����ߴ�
C��������� D�����ж�
(3)�ӱ���ʵ�����������û����Һ������Ũ���ɴ�С��˳����__________________________�����У�[A��]��________ mol��L��1(���������Ƽ��㣬�ش�ȷֵ�������һ��Ҫ����)��
(4)����ʵ���У�HA��NaOH��Һ���ǰ[HA]________(�����������������)[NaOH]��b________(�����������������)7��
��ѧѧ���еĻ�ѧƽ�⡢����ƽ�⡢ˮ��ƽ����ܽ�ƽ���������������ԭ������ش��������⣺
��1�������£�ȡpH��2������ʹ�����Һ��100 mL�������зֱ����������Zn������Ӧ����������Һ��pH�仯��ͼ��ʾ����ͼ�б�ʾ������Һ��pH�仯���ߵ���________���A����B�������������вμӷ�Ӧ��Zn������Ϊm1��������Һ�вμӷ�Ӧ��Zn������Ϊm2����m1________m2��ѡ���������������������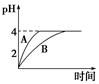
��2����֪������Cu��OH��2��Ksp��2��10��20����֪������ijCuSO4��Һ��c��Cu2������0.02 mol��L��1�����Ҫ����Cu��OH��2��������Ӧ������ҺpH����________��Ҫʹ0.2 mol��L��1��CuSO4��Һ��Cu2��������Ϊ��ȫ��ʹCu2��Ũ�Ƚ���ԭ����ǧ��֮һ������Ӧ����Һ���NaOH��Һ��ʹ��ҺpHΪ________��
��3��10 ��ʱ����NaHCO3������Һ����ø���Һ��pH�������±仯��
| �¶�/�� | 10 | 20 | 30 | ������к���ȴ��50 �� |
| pH | 8.3 | 8.4 | 8.5 | 8.8 |
�ڷ�������ҺpH�����ԭ��ʱ����ͬѧ��Ϊ�������¶�HCO3-��ˮ��̶��������£���ͬѧ��Ϊ����Һ�������¶�NaHCO3���ȷֽ�����Na2CO3��CO32-ˮ��̶ȴ���HCO3-���¡��������һ����ʵ�鷽����������λͬѧ��˵�������У���������������ͽ��ۣ�________________________________________________________________________��

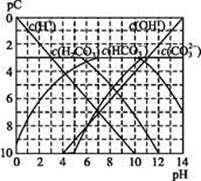
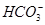 ����ʹ����ѪҺpH������7.35~7.45�����á������õ������Һ�е�ƽ�����:������������������������������(�����ӷ���ʽ��ʾ)��
����ʹ����ѪҺpH������7.35~7.45�����á������õ������Һ�е�ƽ�����:������������������������������(�����ӷ���ʽ��ʾ)��  ������������
������������  ��������������10%��������Ƽ�ʵ��֤����ͬѧ�Ĺ۵��Ƿ���ȷ��
��������������10%��������Ƽ�ʵ��֤����ͬѧ�Ĺ۵��Ƿ���ȷ��  )="0.075" mol��L-1����t1ʱ����������ϵ�м���100 mL 0.125 mol��L-1 Na2CO3��Һ,��ʽ����˵���Ƿ��г���������
)="0.075" mol��L-1����t1ʱ����������ϵ�м���100 mL 0.125 mol��L-1 Na2CO3��Һ,��ʽ����˵���Ƿ��г���������