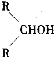��Ŀ����
����Ŀ����ѧҪ�о���κ�������Ч�ؽ��н�������Ŀ������á�
��1�����·��������ڹ�ҵұ������������_______��
a���������AlCl3��ȡ������ b�����MgCl2��Һ��ȡ����þ
c����CO��ԭ����ʯ����ȡ������ d����ⱥ��ʳ��ˮ����ȡ������
��2����ͼ��ʾװ�ý���ʵ�顣

��ʵ������Ӧ���ң��������䣬©���·��к���������������
�ɴ��жϸ÷�Ӧ��________������ȡ����ȡ�����Ӧ���䷴Ӧ�Ļ�ѧ����ʽ��___________________________________________��
���𰸡� c ���� 2Al+Fe2O3![]() 2Fe+Al2O3
2Fe+Al2O3
����������1��a��AlCl3�ǹ��ۻ����Ӧ���õ������Al2O3��ȡ��������ѡ��a����b��Ӧ�õ�����ڵ�MgCl2��ȡ����þ��ѡ��b����c�����DZȽϻ��õĽ������ڹ�ҵ�Ͼ�����CO��ԭ����ʯ����ȡ�������ģ�ѡ��c��ȷ��d���������ʳ������ȡ�����ƣ�ѡ��d����ѡc��(2) ��ʵ�������֪���÷�ӦΪ���ȷ�Ӧ����������������Ӧ��������������������Ӧ�Ļ�ѧ����ʽΪ2Al+Fe2O3![]() 2Fe+Al2O3��
2Fe+Al2O3��

����Ŀ��ʵ�����Ʊ�������ķ�Ӧ���£�NaBr+H2SO4==HBr+NaHSO4 ��
R-OH+HBr![]() R-Br+H2O��
R-Br+H2O��
���ܴ��ڵĸ���Ӧ�У�����Ũ����Ĵ�������ˮ����ϩ���ѣ�Br-��Ũ��������ΪBr2�ȡ��й������б����£�
�Ҵ� | ������ | ������ | 1-�嶡�� | |
�ܶ�/g��cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
�е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
��ش��������⣺
��1�����������1-�嶡����Ʊ�ʵ���У���������������õ����� ��
a��Բ����ƿb������ƿc����ƿd����Ͳ
��2���������ˮ���� ������ڡ��������ڡ���С�ڡ�����Ӧ�Ĵ�����ԭ������
��3����1-�嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã������� ����ϲ㡱�����²㡱���ֲ㡱����
��4���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ������ȷ������
a��ˮ�Ƿ�Ӧ�Ĵ��� b������Br2������
c������HBr�Ļӷ�d�����ٸ�����ϩ���ѵ�����
��5�����Ʊ�������ʱ�����ñ߷�Ӧ����������ķ�������ԭ���� �������Ʊ�1-�嶡��ʱȴ���ܱ߷�Ӧ�����������ԭ���� ��