��Ŀ����
��15�֣��ߴ�������������Fe2O3�����ִ����ӹ�ҵ����Ҫ���ϡ�ʵ��������������������Ҫ�ɷ�ΪFe2O3��FeO��������SiO2�����ʣ�Ϊԭ���Ʊ��ߴ��������IJ������£�

�ش��������⣺
 ��1������ʵ�����漰�ķ�Ӧ�У���һ����Ӧ�����ڻ��Ϸ�Ӧ��������������ԭ��Ӧ��д���÷�Ӧ�����ӷ���ʽ�� ��
��1������ʵ�����漰�ķ�Ӧ�У���һ����Ӧ�����ڻ��Ϸ�Ӧ��������������ԭ��Ӧ��д���÷�Ӧ�����ӷ���ʽ�� ��
��2��ʵ��������18.4mol��L-1��Ũ��������100mL 5.0mol��L-1������
��Һ�����õIJ���������ͷ�ιܡ���Ͳ���ձ����������⣬����
����д�������ƣ���
��3��ijͬѧ����ͼ��ʾװ�ý��й��˲�����
����ָ�����еĴ���֮���� ��
�ڹ��˺�ϴ�ӹ����������������ķ����� ��
��4��ijͬѧ����ͼ��ʾװ�ã�β������װ��δ������ʵ������ҺY��ͨ��NH3��CO2
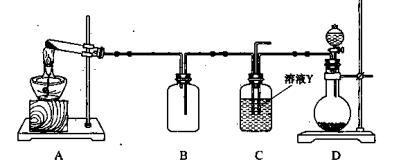
������Ϊʵ�����Ʊ�NH3��CO2�ı�ѡҩƷ��
a.NH4Cl b.CaCO3����״�� c.Ca��OH��2 d.NaOH
e.Ũ��ˮ f.ϡ���� g.ϡ����
������װ��A�����Թ�������ҩƷ�����ѡ��Ϊ �� ����ҩƷ�����գ���װ��D��ҩƷ�����ѡ��Ϊ �� ����ҩƷ�����գ���
�����и����Ʊ�ʵ���У�Ҳ������װ��D��������ɵ��� ������ţ���
E��Zn��ϡ���ᷴӦ�Ʊ�H2
��д������װ��A�����Թ�����������Ӧ�Ļ�ѧ����ʽ ��
����ͨ��һ������NH3��CO2��װ��C������Һ��ֻ����S��N��H��O����Ԫ�ء���pH��ֽ�ⶨ����ҺpH�ķ����� ��������Һ�����ԣ�����Һ�е�NH+4��SO2-4�����ʵ���Ũ�ȼ��������ϵΪ �������ӵ�Ũ���÷���[NH+4]��[SO2-4]��ʾ��

�ش��������⣺
 ��1������ʵ�����漰�ķ�Ӧ�У���һ����Ӧ�����ڻ��Ϸ�Ӧ��������������ԭ��Ӧ��д���÷�Ӧ�����ӷ���ʽ�� ��
��1������ʵ�����漰�ķ�Ӧ�У���һ����Ӧ�����ڻ��Ϸ�Ӧ��������������ԭ��Ӧ��д���÷�Ӧ�����ӷ���ʽ�� ����2��ʵ��������18.4mol��L-1��Ũ��������100mL 5.0mol��L-1������
��Һ�����õIJ���������ͷ�ιܡ���Ͳ���ձ����������⣬����
����д�������ƣ���
��3��ijͬѧ����ͼ��ʾװ�ý��й��˲�����
����ָ�����еĴ���֮���� ��
�ڹ��˺�ϴ�ӹ����������������ķ����� ��
��4��ijͬѧ����ͼ��ʾװ�ã�β������װ��δ������ʵ������ҺY��ͨ��NH3��CO2

������Ϊʵ�����Ʊ�NH3��CO2�ı�ѡҩƷ��
a.NH4Cl b.CaCO3����״�� c.Ca��OH��2 d.NaOH
e.Ũ��ˮ f.ϡ���� g.ϡ����
������װ��A�����Թ�������ҩƷ�����ѡ��Ϊ �� ����ҩƷ�����գ���װ��D��ҩƷ�����ѡ��Ϊ �� ����ҩƷ�����գ���
�����и����Ʊ�ʵ���У�Ҳ������װ��D��������ɵ��� ������ţ���
| A��MnO2��Ũ���ᷴӦ�Ʊ�Cl2 |
| B��Cu��Ũ���ᷴӦ����SO2 |
| C����KMnO4�ֽ���O2 |
| D���Ҵ������ᷴӦ�Ʊ��������� |
��д������װ��A�����Թ�����������Ӧ�Ļ�ѧ����ʽ ��
����ͨ��һ������NH3��CO2��װ��C������Һ��ֻ����S��N��H��O����Ԫ�ء���pH��ֽ�ⶨ����ҺpH�ķ����� ��������Һ�����ԣ�����Һ�е�NH+4��SO2-4�����ʵ���Ũ�ȼ��������ϵΪ �������ӵ�Ũ���÷���[NH+4]��[SO2-4]��ʾ��

��

��ϰ��ϵ�д�
�����Ŀ
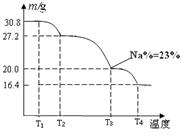
 2 NH3 (g); ��H <0, �÷�ӦӦ��ȡ�����������ǣ� ��
2 NH3 (g); ��H <0, �÷�ӦӦ��ȡ�����������ǣ� ��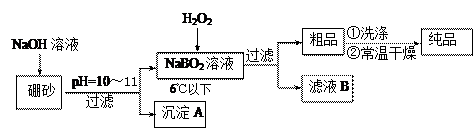
 ���н�����NaBO2 �����Ʒ���Ƶ��������� �� ���� �����ߡ��������͡����䡱����
���н�����NaBO2 �����Ʒ���Ƶ��������� �� ���� �����ߡ��������͡����䡱����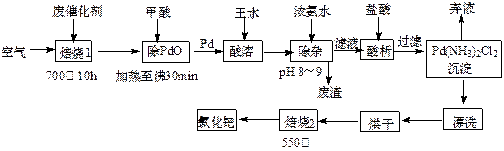
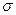 ����1��
����1��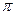 ��
�� >CO2
>CO2 5SH>C2H5OH
5SH>C2H5OH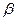 -ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
-ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����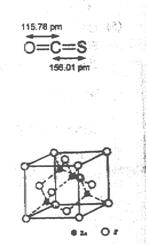

 ��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���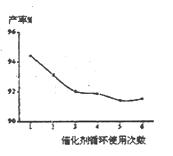
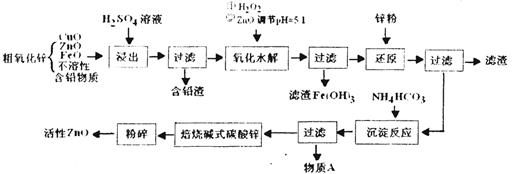
 ������������Ư������������������Ϣ������л�ѧ����ʽ��
������������Ư������������������Ϣ������л�ѧ����ʽ��