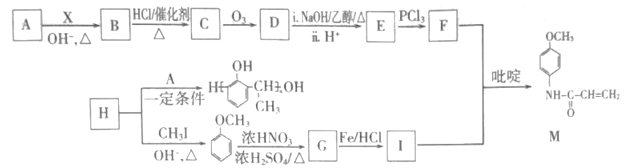
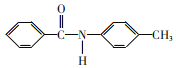
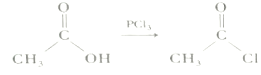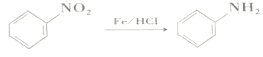��Ŀ����
����Ŀ��������һ�����͵���ɫ��Դ������һ����Ҫ�Ļ���ԭ�ϡ�
��1������ȼ����ֵ�ߡ�ʵ���ã��ڳ��³�ѹ�£�1gH2��ȫȼ������Һ̬ˮ���ų�142.9kJ��������H2ȼ�յ��Ȼ�ѧ����ʽΪ_____
��2�������Ǻϳɰ�����Ҫԭ�ϣ��ϳɰ���Ӧ���Ȼ�����ʽ���£�N2(g)��3H2(g)![]() 2NH3(g)����H����92.4kJ/mol
2NH3(g)����H����92.4kJ/mol
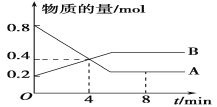
��һ�������£�һ������N2��H2��Ӧ�ﵽƽ��ı�ijһ�����������Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ�����м����������Ӧ���ʱ仯��ʱ�����____(���0��t1��)��
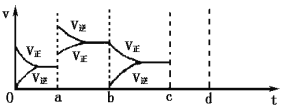
��������Ϊ��߷�Ӧ���ʺ�H2��ת���ʣ����д�ʩ���е���___������ĸ����
A����װ���г������N2 B����ʱ���ϳɵİ�����װ���з������
C��ʹ�ø���Ч�Ĵ��� D�������¶�
���¶�ΪT��ʱ����2amolH2��amolN2����0.5L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50%����Ӧ��ƽ�ⳣ��Ϊ____��
���𰸡�2H2(g)��O2(g)��2H2O(l) ��H����571.6 kJ/mol t4��t5 A ![]()
��������
(1)1gH2��ȫȼ������Һ̬ˮ���ų�142.9kJ��������2g������ȫȼ������Һ̬ˮ�ų�571.6kJ������������ȼ���Ȼ�ѧ����ʽΪ2H2(g)+O2(g)=2H2O(l)��H=571.6kJ/mol��
�ʴ�Ϊ��2H2(g)+O2(g)=2H2O(l)��H=571.6kJ/mol��
(2)�ٴ�����ͬ�ȳ̶ȵĸı����淴Ӧ���ʣ��������淴Ӧ������Ȼ��ȣ����Լ��������ʱ���Ϊt4t5��
�ʴ�Ϊ��t4t5��
��A. ��װ���г������N2����ѧ��Ӧ���ʼӿ����������ת���ʣ���A��ȷ��
B. ��ʱ���ϳɵİ�����װ���з���������������ת���ʣ����ǻ�ѧ��Ӧ���ʼ�������B����
C. ʹ�ø���Ч�Ĵ��������ѧ��Ӧ���ʣ���������ת���ʲ��䣬��C����
D. �����¶�ƽ�������ƶ�����������ת���ʼ�С����D����
��ѡA��
���¶�ΪT��ʱ����2amolH2��amolN2����0.5L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50%����ʼʱc(N2)=2amol/L��c(H2)=4amol/L��ƽ��ʱ����c(N2)=2amol/L��50%=amol/L��
���淴Ӧ N2(g)+3H2(g)2NH3(g)
��ʼ(mol/L) 2a 4a0
��Ӧ(mol/L) a 3a2a
ƽ��(mol/L) a a2a
��Ӧ��ƽ�ⳣ��K
�ʴ�Ϊ��![]() ��
��

����Ŀ�������廷������ȫ��������������Ž���һ�����и����е����ʣ���������ͼ����Ӱ���ֹ�ϵ����(����)

�� | �� | �� | �� | |
A | NaCl | K2SO4 | KCl | (NH4)2SO4 |
B | Na2SO4 | K2SO4 | KCl | NH4Cl |
C | NaCl | K2SO4 | KCl | NH4Cl |
D | Na2SO4 | K2SO4 | KCl | (NH4)2SO4 |
A.AB.BC.CD.D