��Ŀ����
����Ŀ����������(NaClO2)��һ����Ҫ�ĺ�������������Ҫ����ˮ�������Լ�ɰ�ǡ���֬��Ư����ɱ����ij��ѧ��ȤС��ͬѧչ����Ư����������(NaClO2)���о���
��֪:NaClO2������Һ���¶ȵ���38 ��ʱ�����ľ�����NaClO2��3H2O������38 ��ʱ�����ľ�����NaClO2������60 ��ʱNaClO2�ֽ��NaClO3��NaCl��Ba(ClO)2������ˮ��
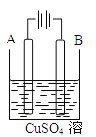
������ͼ��ʾװ�ý���ʵ�顣

(1)����a������Ϊ____��װ�âٵ�������____��װ�â۵�������______��
(2)װ�â��в���ClO2���漰��Ӧ�Ļ�ѧ����ʽΪ________________��װ�â��з�Ӧ����NaClO2�Ļ�ѧ����ʽΪ_______________��
(3)��װ�âܷ�Ӧ�����Һ��þ���NaClO2�IJ�������Ϊ:�ټ�ѹ��55 �������ᾧ���ڳ��ȹ��ˣ���______���ܵ���60 �����õ���Ʒ�������ȥ���е���ˮԡ�����ܵ��²�Ʒ�л��е�������________��
(4)���ʵ���������NaClO2�����Ƿ�������Na2SO4��������������:ȡ����������������ˮ��_____________________��
(5)Ϊ�˲ⶨNaClO2��Ʒ�Ĵ��ȣ�ȡ�����ֲ�Ʒ10.0 g����ˮ���1 L��Һ��ȡ��10 mL��Һ����ƿ�У��ټ��������ữ��KI��Һ����ַ�Ӧ��(NaClO2����ԭΪCl-�����ʲ��μӷ�Ӧ)������2~3�ε�����Һ����0.20 mol��L-1Na2S2O3��Һ�ζ����ﵽ�ζ��յ�ʱ��ȥ��Һ20.00 mL���Լ���NaClO2��Ʒ�Ĵ���_____��(��ʾ:2Na2S2O3+I2![]() Na2S4O6+2NaI)
Na2S4O6+2NaI)
���𰸡� ������ƿ ���ն����ClO2���壬��ֹ��Ⱦ���� ��ֹ����(������ȫƿ��������ȷ˵��) 2NaClO3+Na2SO3+H2SO4(Ũ)![]() 2ClO2��+2Na2SO4+H2O 2NaOH+2ClO2+H2O2
2ClO2��+2Na2SO4+H2O 2NaOH+2ClO2+H2O2![]() 2NaClO2+2H2O+O2 ��38 ��~60 �����ˮϴ�� NaClO3��NaCl �μӼ���BaCl2��Һ�����а�ɫ�������֣�����Na2SO4�����ް�ɫ�������֣���Na2SO4 90.5%
2NaClO2+2H2O+O2 ��38 ��~60 �����ˮϴ�� NaClO3��NaCl �μӼ���BaCl2��Һ�����а�ɫ�������֣�����Na2SO4�����ް�ɫ�������֣���Na2SO4 90.5%
��������(1)����a������Ϊ������ƿ ��װ������NaOH��Һ�����������ն����ClO2���壬��ֹ��Ⱦ������ClO2����װ������������Ӧʹװ����ѹǿ���ͣ����ܷ���������װ����������Ϊ��ֹ������
(2)�������ƾ��л�ԭ�ԣ��ڷ�Ӧ������ԭ����װ�����в���ClO2�ķ�Ӧ����������������Һ��������������Ϊ�����ƣ���������ԭΪ�������ȣ���Ӧ�Ļ�ѧ����ʽӦΪ��2NaClO3+Na2SO3+H2SO4=2ClO2��+2Na2SO4+H2O��װ������H2O2�ڼ�������������ClO2����Ӧ����NaClO2�Ļ�ѧ����ʽΪ2NaOH+2ClO2+H2O2![]() 2NaClO2+2H2O+O2��
2NaClO2+2H2O+O2��
(3)����Һ����ȡ���壬һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����Ϊ��ֹ��������NaClO23H2O��Ӧ���ȹ��ˣ�����Ŀ��Ϣ��֪��Ӧ�����¶�38����60������ϴ�ӣ�����60�����NaClO2������Һ���¶ȸ���60 ��ʱNaClO2�ֽ��NaClO3��NaCl���������ȥ���е���ˮԡ�����ܵ��²�Ʒ�л��е�������NaClO3��NaCl��
(4)������NaClO2�����Ƿ�������Na2SO4��ʵ��ֻҪ���龧���ˮ��Һ���Ƿ���SO42-�����������������:ȡ����������������ˮ���μӼ���BaCl2��Һ�����а�ɫ�������֣�����Na2SO4�����ް�ɫ�������֣���Na2SO4��
(5)ȡ�����ϳɲ�Ʒ10g����ˮ���1L��Һ��ȡ��10mL��Һ����ƿ�У��ټ��������ữ��KI��Һ������ClO2-+4I-+4H+=Cl-+2I2+2H2O����ַ�Ӧ�����2��3�ε�����Һ����Һ��������0.20mol/L Na2S2O3��Һ�ζ���������2Na2S2O3+I2�TNa2S4O6+2NaI����ɫ��Ϊ��ɫ��
�ɵ÷�Ӧ�Ĺ�ϵʽΪ��ClO2-��2I2��4Na2S2O3����n(Na2S2O3)=0.20mol/L��0.02L=0.004mol��
��ClO2-��2I2��4Na2S2O3
1 4
n(ClO2-) 0.004mol n(ClO2-)=0.001mol������1L��Һ�к��У�n(NaClO2)=0.001mol��100=0.1mol����10g�ϳɲ�Ʒ�к��У�m(NaClO2)=0.1mol��90.5g/mol=0.905g��NaClO2��Ʒ�Ĵ���Ϊ![]() ��100%=90.5%��
��100%=90.5%��