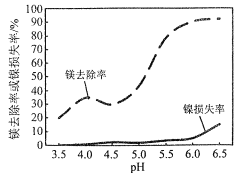
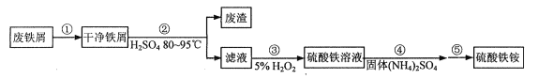
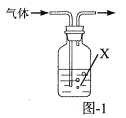
��Ŀ����
����Ŀ������һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����NH![]() ��Cl����Mg2+��Ba2+��CO
��Cl����Mg2+��Ba2+��CO![]() ��SO
��SO![]() ����ȡ���� 100 mL ��Һ��������ʵ�飺
����ȡ���� 100 mL ��Һ��������ʵ�飺
(1)��һ�ݼ����� NaOH ��Һ���Ⱥ��ռ������� 0.04 mol��
(2)�ڶ��ݼ����� BaCl2 ��Һ�ø������ 6.27 g������������ϴ�ӡ������������Ϊ 2.33 g��
(3)�����ݼ��� AgNO3 ��Һ�г�����������������ʵ�飬�����Ʋⲻ��ȷ����
A.100 mL ��Һ�к� 0.04 mol NH![]()
B.100 mL ��Һ�к� 0.01 mol SO42-�� 0.02 mol CO32-
C.K����Cl�����ܴ���
D.һ��������Ba2+��Mg2+
���𰸡�C
��������
(1)��һ�ݼ����� NaOH ��Һ���Ⱥ��ռ������� 0.04 mol��������ΪNH3���Ӷ��ó�ԭ��Һ�к�NH4+0.04mol��
(2)�ڶ��ݼ����� BaCl2 ��Һ�ø������ 6.27 g������������ϴ�ӡ������������Ϊ 2.33 g����BaSO4Ϊ2.33g��![]() =0.01mol��BaCO3Ϊ6.27g-2.33g=3.94g��
=0.01mol��BaCO3Ϊ6.27g-2.33g=3.94g��![]() =0.02mol��
=0.02mol��
(3)��Ϊ����SO42-��CO32-ʱ��������BaCl2��������Cl-�����Լ��� AgNO3 ��Һ�г������������ܿ϶�ԭ��Һ���Ƿ���Cl-��
A�������Ϸ�����100 mL��Һ�к�0.04 mol NH![]() ��A��ȷ��
��A��ȷ��
B����������ķ�������㣬100 mL��Һ�к�0.01 mol SO42-��0.02 mol CO32-��B��ȷ��
C���������ӹ��棬��Һ�к���NH![]() ��CO
��CO![]() ��SO
��SO![]() ����һ��������Mg2+��Ba2+�����ݵ���غ㣬�������������������Ϊ0.04mol���������������������Ϊ0.06mol����K������Ϊ0.02mol�����ܿ϶�Cl���Ƿ���ܣ�C����ȷ��
����һ��������Mg2+��Ba2+�����ݵ���غ㣬�������������������Ϊ0.04mol���������������������Ϊ0.06mol����K������Ϊ0.02mol�����ܿ϶�Cl���Ƿ���ܣ�C����ȷ��
D����C�з�����֪��һ��������Ba2+��Mg2+��D��ȷ��
��ѡC��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�