��Ŀ����
(1)ij������ȼ�ϵ�����ù�����������մ�������ʣ������Ϸ����ĵ缫��Ӧ�ֱ�Ϊ��A����2H2��2O2����4e��=2H2O��B����O2��4e��=2O2��
��A���ǵ�ص�________�������ӴӸü�________(����롱��������)��
(2)�����Ի�ѧ��Ӧ��2Zn��O2��4H��=2Zn2����2H2OΪ�������һ��ԭ��أ�������������Ϊ������������Դ�����ǿ�������ѪҺ������һ��Ũ�ȵ�O2��H����Zn2�����й�������ԭ��صĸ���������________�������ĵ缫��ӦΪ__________________��
(3)��һ�鴿����пƬ����װ��ϡ������ձ���ɹ۲쵽пƬ�������ݣ���ƽ�в���һ��ͭƬ���ɹ۲쵽ͭƬ��________(��С���û�С�)���ݲ��������õ��߰�пƬ��ͭƬ�������������һ��ԭ��أ������ĵ缫��ӦʽΪ____________________��
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�[��ѧ-ѡ��3�����ʽṹ������]
���ո���ʱ�ڣ�Ⱥ�����������������������ͻ��ϣ���ʱȺ���������ɹ��ص����ʯ��ĥ���Ƴɵġ����ʯ��ָ�����������ο�����к��ơ������衢���ȡ�����Ԫ�ء�
(1)��Ԫ�ػ�̬ԭ�ӵļ۵��ӹ����ʾʽΪ____________��
(2)���������У�����δ�ɶԵ�������ͬ�Ľ���Ԫ����________�֡�
(3)Na+��Ne��Ϊ�ȵ����壬������I2(Na)________I1(Ne)���>����<������
(4)����֪���ж��ֺ����ᣬ�����ƽ�ⳣ�����£�
��ѧʽ | HClO4 | HClO3 | HClO2 | HClO |
Ka | 1��1010 | 1��101 | 1��10-2 | 4��10-8 |
HClO4�Ľṹ��ʽΪ_________��HClO3��Clԭ�ӵ��ӻ��������Ϊ________��HClO2�к��еĹ��ۼ�����Ϊ__________�����ϼ��ֺ����������ǿ����ͬ����ԭ��Ϊ______________��
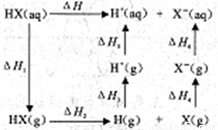
����±�ᣨHX���ĵ��������ͼ����H1�ͦ�H2�ĵݱ���ɶ���HF>HCl>HBr>HI�����Ц�H1(HF)�ر���ԭ��Ϊ_________��Ӱ�즤H2�ݱ������Ϊ______________��
(5)������Ϊ�����������壬��������a=q nm������Ħ������ΪMg��mol-1��ԭ�Ӱ뾶Ϊr pm������٤��������ֵΪNA���������ʵ��ܶ�Ϊ________g��cm-3����ʽ���ɣ���ͬ������������ԭ�ӵ����ռ��������İٷ���Ϊ____________��


 6Cu��SO2�����÷�Ӧ����������________��������19.2 g Cu ʱ����Ӧ��ת�Ƶĵ���Ϊ________ mol��
6Cu��SO2�����÷�Ӧ����������________��������19.2 g Cu ʱ����Ӧ��ת�Ƶĵ���Ϊ________ mol��
 Ϊ��ʼԭ�ϣ����Լ���ѡ���Ʊ�
Ϊ��ʼԭ�ϣ����Լ���ѡ���Ʊ� �ĺϳ�·��_________________��
�ĺϳ�·��_________________��
