��Ŀ����
ͭ��������������Ԫ�أ�Ҳ����������ʹ�õĽ���֮һ��ͭ��������ʹ�öԹ��������������涼��������Զ��Ӱ�졣
��1��Ϊ�˱��������ͽ�Լ��Դ��ͨ������H2O2��ϡ����Ļ����Һ�ܳ��Ͼ�ӡˢ��·���е�ͭ������ʵ��ͭ�Ļ������á�д��H2O2��ϡ����Ļ����Һ�����ͭ��Ӧ�����ӷ���ʽ��____________________________________��
��2����ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu2O��Cu2S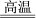 6Cu��SO2�����÷�Ӧ����������________��������19.2 g Cu ʱ����Ӧ��ת�Ƶĵ���Ϊ________ mol��
6Cu��SO2�����÷�Ӧ����������________��������19.2 g Cu ʱ����Ӧ��ת�Ƶĵ���Ϊ________ mol��
��3���о���ѧϰС���á���ӵ��������ⶨij������CuSO4��5H2O(��������I����Ӧ������������)�ĺ�������ȡa g������� 100 mL ��Һ��ȡ��25.00 mL��Һ�������еμ� KI ��Һ���а�ɫ�������ɣ������ķ�ӦΪ2Cu2����4I��===2CuI����I2���������μ� KI ��Һ���������ٲ�������Ӧ���ɵ� I2 ��V mL c mol��L-1Na2S2O3��Һǡ����ȫ��Ӧ�������ķ�ӦΪI2��2Na2S2O3===2NaI��Na2S4O6������������CuSO4��5H2O����������Ϊ_________________��
 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
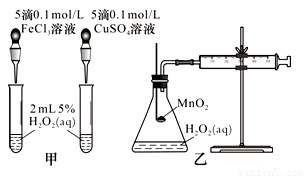
��ĩ�����ϵ�д�Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ�鷽������̽����
��1��ȡ�����ʵ���Ũ�ȡ��������H2O2��Һ�ֱ����H2O2�ķֽ�ʵ�飬ʵ�鱨�����±���ʾ������ͽ����ԣ���
��� | �¶�/�� | ���� | ���� | ���� |
1 | 40 | FeCl3��Һ | ||
2 | 20 | FeCl3��Һ | ||
3 | 20 | MnO2 | ||
4 | 20 | �� |
��ʵ��1��2�о�����________________��H2O2�ֽ����ʵ�Ӱ�졣
��ʵ��2��3��Ŀ����_______________��
��2��������֪��Cu2����H2O2�ֽ�Ҳ�д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч������С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
�ٶ��Է�������ͼ��ͨ���۲�________________�����ԱȽϵó����ۡ���ͬѧ�����CuSO4��Һ��ΪCuCl2��Һ����������������_______________��
�ڶ�����������ͼ����ʾ��ʵ��ʱ���ռ���40 mL����Ϊ��������������Ӱ��ʵ������أ�ʵ������Ҫ������������_____________��
��3�����Ը��������Һ�Ͳ�����Һ�ɷ�����Ӧ��2KMnO4��5H2C2O4��3H2SO4=K2SO4��2MnSO4��8H2O��10CO2����ʵ��ʱ���ֿ�ʼ��Ӧ���ʽ�������Һ��ɫ�����ԣ���һ��ʱ���ͻȻ��ɫ����Ӧ�������Լӿ졣�Դ�չ�����ۣ�
��ijͬѧ��ΪKMnO4��H2C2O4�ķ�Ӧ�� �ȷ�Ӧ������_______________��
�ڴ�Ӱ�컯ѧ��Ӧ���ʵ����ؿ�������Ϊ��������____________________��Ӱ�졣Ҫ֤����IJ��룬ʵ�鷽���� ��



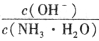 ��_______(����� ����С������ȷ��������
��_______(����� ����С������ȷ��������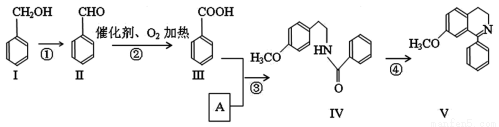
 д���������������Ģ��Ľṹ��ʽ
д���������������Ģ��Ľṹ��ʽ 