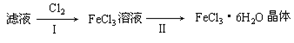��Ŀ����
��15�֣��ۺ��������ֳƾ�������ѧʽΪ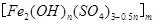 ���㷺������ˮ������ʵ�����������᳧��������Ҫ�ɷ�Ϊ���������P����FeS��SiO2�ȣ��Ʊ��������̷���FeSO4��7H2O ���������£�
���㷺������ˮ������ʵ�����������᳧��������Ҫ�ɷ�Ϊ���������P����FeS��SiO2�ȣ��Ʊ��������̷���FeSO4��7H2O ���������£�
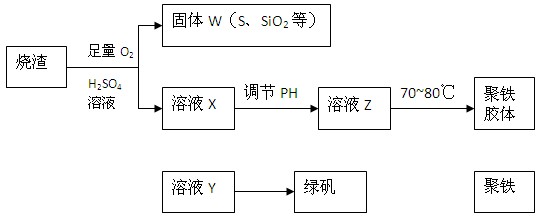
��1����֤����W���պ���������庬��SO2�ķ�����___��
��2���Ʊ��̷�ʱ������ҺX�м������___����ַ�Ӧ��_____�����õ���ҺY���پ�Ũ�����ᾧ�Ȳ���õ��̷���
��3����ҺZ��pHӰ�����������������������pH��ֽ�ⶨ��ҺpH�IJ�������Ϊ____������ҺZ��pHƫС�������¾�����������������ƫ_____��
��4���Ŵ����̷����տ����̷��ͣ�Ҳ����ˮ�������ᣩ�ͺ�ɫ���ϣ�Fe2O3������д���йصĻ�ѧ����ʽ��
��
��5���̷����������·���������ɫ���ϣ�Fe2O3��,�������������ǣ���5560kg�̷���Ħ������Ϊ278 g/mol������ˮ�У�������������������Һǡ����ȫ��Ӧ�����������������裬�������ɫ���壻������ɫ�����м���16680 kg �̷���560 kg���ۣ����������������裬��Ӧ��ɺ��д���Fe2O3�����ڽ����������Գ�����ʽ���������˺������������յú�ɫ���ϡ���������Һ������ֻ�������ƺ����������������Ͽ�������ɫ����____________________kg��
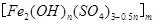 ���㷺������ˮ������ʵ�����������᳧��������Ҫ�ɷ�Ϊ���������P����FeS��SiO2�ȣ��Ʊ��������̷���FeSO4��7H2O ���������£�
���㷺������ˮ������ʵ�����������᳧��������Ҫ�ɷ�Ϊ���������P����FeS��SiO2�ȣ��Ʊ��������̷���FeSO4��7H2O ���������£�
��1����֤����W���պ���������庬��SO2�ķ�����___��
��2���Ʊ��̷�ʱ������ҺX�м������___����ַ�Ӧ��_____�����õ���ҺY���پ�Ũ�����ᾧ�Ȳ���õ��̷���
��3����ҺZ��pHӰ�����������������������pH��ֽ�ⶨ��ҺpH�IJ�������Ϊ____������ҺZ��pHƫС�������¾�����������������ƫ_____��
��4���Ŵ����̷����տ����̷��ͣ�Ҳ����ˮ�������ᣩ�ͺ�ɫ���ϣ�Fe2O3������д���йصĻ�ѧ����ʽ��
��
��5���̷����������·���������ɫ���ϣ�Fe2O3��,�������������ǣ���5560kg�̷���Ħ������Ϊ278 g/mol������ˮ�У�������������������Һǡ����ȫ��Ӧ�����������������裬�������ɫ���壻������ɫ�����м���16680 kg �̷���560 kg���ۣ����������������裬��Ӧ��ɺ��д���Fe2O3�����ڽ����������Գ�����ʽ���������˺������������յú�ɫ���ϡ���������Һ������ֻ�������ƺ����������������Ͽ�������ɫ����____________________kg��
��1��������ͨ��Ʒ����Һ�У���Ʒ����ɫ�����Ⱥ��ֱ�죬ע���ж�������
��2������ ����
��3������ֽ�ŵ���������,�ò�����պȡ��Һ,�㵽��ֽ������,Ȼ�������ɫ���Ա� ��
��4��2FeSO4��7H2O Fe2O3��SO2����SO3����14H2O��SO3��H2O=H2SO4
Fe2O3��SO2����SO3����14H2O��SO3��H2O=H2SO4
��5��4000
��2������ ����
��3������ֽ�ŵ���������,�ò�����պȡ��Һ,�㵽��ֽ������,Ȼ�������ɫ���Ա� ��
��4��2FeSO4��7H2O
 Fe2O3��SO2����SO3����14H2O��SO3��H2O=H2SO4
Fe2O3��SO2����SO3����14H2O��SO3��H2O=H2SO4��5��4000
��1�������������Ư���Կ���ʹƷ����Һ��ɫ������Ư�ײ��Ǻ��ȶ������Ⱥ��ֿɻָ�ԭ����ɫ��
��2����ҺX�к���Fe3��,�����ȥ,���ֲ��������µ�����,��ѡ������,����������ͨ�����˳�ȥ.
(3)������Һ��pHֵ��һ����������ʪpH��ֽ��Ҳ���ܲ��뵽��Һ�У���ȷ�ķ����ǽ���ֽ�ŵ���������,�ò�����պȡ��Һ,�㵽��ֽ������,Ȼ�������ɫ���Աȡ�����ҺZ��pHƫС��˵�����Խ�ǿ�����ǵľ�����������ƫ�٣������ƫ�࣬���������������ƫ�͡�
��4����������ɵø÷�Ӧ����������ԭ��Ӧ���ж����������ɣ�����ʽΪ
2FeSO4��7H2O Fe2O3��SO2����SO3����14H2O��SO3��H2O=H2SO4��
Fe2O3��SO2����SO3����14H2O��SO3��H2O=H2SO4��
��5�����ݷ���ʽ�ɼ�����Ҫ�������Ƶ����ʵ�����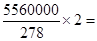 40000mol����ԭ���غ�֪�����Ƶ����ʵ�����20000mol�������������ɵ��������е�SO42��������������̷�����������̷���
40000mol����ԭ���غ�֪�����Ƶ����ʵ�����20000mol�������������ɵ��������е�SO42��������������̷�����������̷���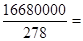 60000mol������������20000mol���ܵ���ԭ����
60000mol������������20000mol���ܵ���ԭ����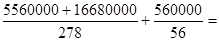 90000mol������ԭ���غ�֪Fe2O3������
90000mol������ԭ���غ�֪Fe2O3������
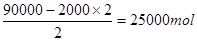 ��������4000kg��
��������4000kg��
��2����ҺX�к���Fe3��,�����ȥ,���ֲ��������µ�����,��ѡ������,����������ͨ�����˳�ȥ.
(3)������Һ��pHֵ��һ����������ʪpH��ֽ��Ҳ���ܲ��뵽��Һ�У���ȷ�ķ����ǽ���ֽ�ŵ���������,�ò�����պȡ��Һ,�㵽��ֽ������,Ȼ�������ɫ���Աȡ�����ҺZ��pHƫС��˵�����Խ�ǿ�����ǵľ�����������ƫ�٣������ƫ�࣬���������������ƫ�͡�
��4����������ɵø÷�Ӧ����������ԭ��Ӧ���ж����������ɣ�����ʽΪ
2FeSO4��7H2O
 Fe2O3��SO2����SO3����14H2O��SO3��H2O=H2SO4��
Fe2O3��SO2����SO3����14H2O��SO3��H2O=H2SO4����5�����ݷ���ʽ�ɼ�����Ҫ�������Ƶ����ʵ�����
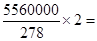 40000mol����ԭ���غ�֪�����Ƶ����ʵ�����20000mol�������������ɵ��������е�SO42��������������̷�����������̷���
40000mol����ԭ���غ�֪�����Ƶ����ʵ�����20000mol�������������ɵ��������е�SO42��������������̷�����������̷���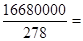 60000mol������������20000mol���ܵ���ԭ����
60000mol������������20000mol���ܵ���ԭ����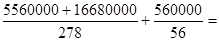 90000mol������ԭ���غ�֪Fe2O3������
90000mol������ԭ���غ�֪Fe2O3������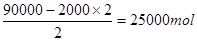 ��������4000kg��
��������4000kg��
��ϰ��ϵ�д�
�����Ŀ
 6SO2��Fe3O4����3 molFeS2�μӷ�Ӧ��ת��������mol����
6SO2��Fe3O4����3 molFeS2�μӷ�Ӧ��ת��������mol���� C��s��=
C��s��= 2Fe��s��+3CO2��g��;��ҵ����16 t Fe2O3��200m3�������з�Ӧ��lСʱ����Fe2O3��ת����Ϊ50%�������ʱ����CO����������Ϊ mol/��L��h����
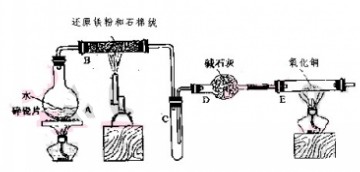
2Fe��s��+3CO2��g��;��ҵ����16 t Fe2O3��200m3�������з�Ӧ��lСʱ����Fe2O3��ת����Ϊ50%�������ʱ����CO����������Ϊ mol/��L��h���� Fe��OH��2+Ni��OH��2���������ƣ� Na2FeO4����һ��������ˮ������������29ͼװ�ÿ�����ȡ�����������ƣ�
Fe��OH��2+Ni��OH��2���������ƣ� Na2FeO4����һ��������ˮ������������29ͼװ�ÿ�����ȡ�����������ƣ�