��Ŀ����
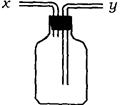
��13�֣�(1)��ͼ��ʾװ�ã�Ϊʵ������ʵ��Ŀ�ģ�����y���ʺ�������ڵ���_____���
A ƿ��ʢҺ�����������Ը�������
B ƿ��ʢҺ��ϴ�Ӽ������Գ�ȥij�����е�����
C ƿ��ʢˮ�����Բ���ij������ˮ����������
D ƿ���������壬��ˮʱ����ɱ��ų�
E �ռ��ܶȱȿ����������
F �ռ��ܶȱȿ���С������
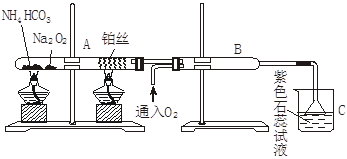
(2) ŨH2SO4��ľ̿�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽ��_______________________��
ͼ�����߿��е�װ�ÿ���������Ũ������ľ̿���ڼ��������·�Ӧ����������������
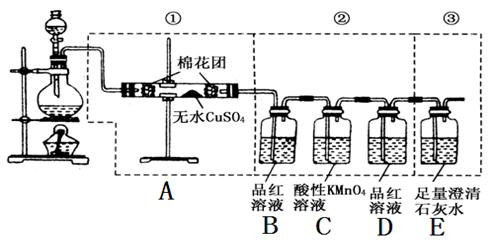
��д���пհס�
��A����ˮ����ͭ������_____________��
��֤��SO2һ�����ڵ�������_____________��
��C������KMnO4��Һ������_____________��
��֤��CO2һ�����ڵ�������_____________��

A ƿ��ʢҺ�����������Ը�������
B ƿ��ʢҺ��ϴ�Ӽ������Գ�ȥij�����е�����
C ƿ��ʢˮ�����Բ���ij������ˮ����������
D ƿ���������壬��ˮʱ����ɱ��ų�
E �ռ��ܶȱȿ����������
F �ռ��ܶȱȿ���С������
(2) ŨH2SO4��ľ̿�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽ��_______________________��
ͼ�����߿��е�װ�ÿ���������Ũ������ľ̿���ڼ��������·�Ӧ����������������

��д���пհס�
��A����ˮ����ͭ������_____________��
��֤��SO2һ�����ڵ�������_____________��
��C������KMnO4��Һ������_____________��
��֤��CO2һ�����ڵ�������_____________��
��1�� A B E (2) C+2H2SO4 CO2��+2SO2��+2H2O
CO2��+2SO2��+2H2O
�ټ���ˮ���� ��B��Ʒ����ɫ �۳�ȥSO2 ��D��Ʒ�첻��ɫ��E�б����
 CO2��+2SO2��+2H2O
CO2��+2SO2��+2H2O�ټ���ˮ���� ��B��Ʒ����ɫ �۳�ȥSO2 ��D��Ʒ�첻��ɫ��E�б����
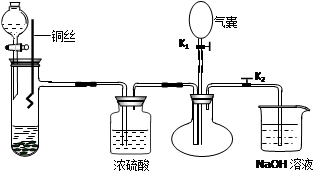
��1����ͼ���Կ���y���ܲ��뵽�Լ�ƿ�ĵײ�����˿���������ijЩ���壬Ҳ��������ȥ�����е�ijЩ���ʣ����������ռ��ܶȴ��ڿ�����ijЩ���塣��ƿ��ʢˮ�����Բ���ij������ˮ�������������������Ӧ��x���ܡ�ͬ��ƿ������������ˮ�����壬��ˮʱ����ɱ��ų���������ͬ��Ӧ��x���ܡ��ռ��ܶȱȿ���С�����壬������Ӧ��x���ܡ�����ȷ�Ĵ���A B E��
��2��Ũ�������ǿ�����ԣ��ڼ��ȵ������¿���������̼������ʽΪC+2H2SO4 CO2��+2SO2��+2H2O������������ˮ������CO2��SO2����֤ˮ��������ˮ����ͭ��CO2�ñ���ʯ��ˮ��SO2��Ʒ����Һ�����ں����߶���Ҫͨ����Һ�����������֤ˮ��������ΪSO2Ҳ����ʹʯ��ˮ����ǣ�����ڼ���CO2֮ǰ����Ҫ�ȼ���SO2�����һ�Ҫ��ȫ��ȥSO2���Ա������CO2�ļ��顣
CO2��+2SO2��+2H2O������������ˮ������CO2��SO2����֤ˮ��������ˮ����ͭ��CO2�ñ���ʯ��ˮ��SO2��Ʒ����Һ�����ں����߶���Ҫͨ����Һ�����������֤ˮ��������ΪSO2Ҳ����ʹʯ��ˮ����ǣ�����ڼ���CO2֮ǰ����Ҫ�ȼ���SO2�����һ�Ҫ��ȫ��ȥSO2���Ա������CO2�ļ��顣
��2��Ũ�������ǿ�����ԣ��ڼ��ȵ������¿���������̼������ʽΪC+2H2SO4
 CO2��+2SO2��+2H2O������������ˮ������CO2��SO2����֤ˮ��������ˮ����ͭ��CO2�ñ���ʯ��ˮ��SO2��Ʒ����Һ�����ں����߶���Ҫͨ����Һ�����������֤ˮ��������ΪSO2Ҳ����ʹʯ��ˮ����ǣ�����ڼ���CO2֮ǰ����Ҫ�ȼ���SO2�����һ�Ҫ��ȫ��ȥSO2���Ա������CO2�ļ��顣
CO2��+2SO2��+2H2O������������ˮ������CO2��SO2����֤ˮ��������ˮ����ͭ��CO2�ñ���ʯ��ˮ��SO2��Ʒ����Һ�����ں����߶���Ҫͨ����Һ�����������֤ˮ��������ΪSO2Ҳ����ʹʯ��ˮ����ǣ�����ڼ���CO2֮ǰ����Ҫ�ȼ���SO2�����һ�Ҫ��ȫ��ȥSO2���Ա������CO2�ļ��顣
��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ

 ʼ����ʱ��pH
ʼ����ʱ��pH ��������ţ�
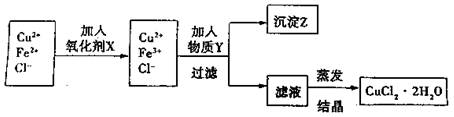
��������ţ� ++H2�����ʵ������ȡCuCl2��Һ����ͬѧ��Ƶ�װ��Ӧ��Ϊ �����ԭ��ء����ء���
++H2�����ʵ������ȡCuCl2��Һ����ͬѧ��Ƶ�װ��Ӧ��Ϊ �����ԭ��ء����ء���


 ��Һ
��Һ
 ����
����