��Ŀ����
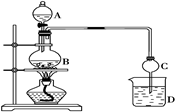
��12�֣�NaClO2�����ޡ��顢ճ����ά��֯���Ư�ס�ʵ�����Ʊ�NaClO2��װ������ͼ��ʾ��
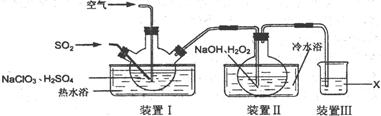
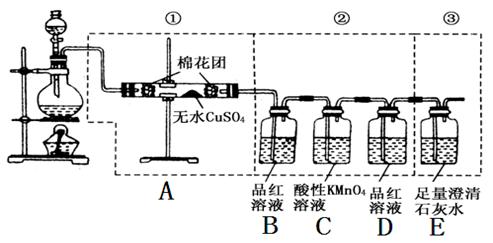
(1)װ��I�����¶���35~55�棬ͨ��SO2��NaClO3��ԭΪClO2���е㣺11�棩����Ӧ������ͨ�������Ŀ�������Ŀ���� ��
(2)װ�â��з�Ӧ����NaClO2�Ļ�ѧ����ʽΪ ����Ӧ�����Һ�������ӳ���ClO2����ClO3����Cl����ClO����OH������ܺ��е�һ���������� ����������ӵķ�����
(3)��֪��NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O���¶ȸ���38��ʱ����������NaClO2���¶ȸ���60��ʱNaClO2�ֽ�����NaClO3��NaCl���벹���װ�â�Ӧ�����Һ�л��NaClO2����IJ������衣
�ټ�ѹ��55�������ᾧ���� ���� ���� ���õ���Ʒ��
(4)װ�â����Լ�XΪ ��

(1)װ��I�����¶���35~55�棬ͨ��SO2��NaClO3��ԭΪClO2���е㣺11�棩����Ӧ������ͨ�������Ŀ�������Ŀ���� ��
(2)װ�â��з�Ӧ����NaClO2�Ļ�ѧ����ʽΪ ����Ӧ�����Һ�������ӳ���ClO2����ClO3����Cl����ClO����OH������ܺ��е�һ���������� ����������ӵķ�����
(3)��֪��NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O���¶ȸ���38��ʱ����������NaClO2���¶ȸ���60��ʱNaClO2�ֽ�����NaClO3��NaCl���벹���װ�â�Ӧ�����Һ�л��NaClO2����IJ������衣
�ټ�ѹ��55�������ᾧ���� ���� ���� ���õ���Ʒ��
(4)װ�â����Լ�XΪ ��
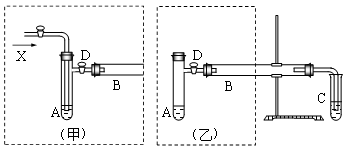
(1)��ClO2���뵽װ�â���з�Ӧ��2�֣�
(2)2NaOH+2ClO2+H2O2��2NaClO2+2H2O+O2(2��)
SO42����1�֣� ȡ������Ӧ�����Һ���ȼ����������ᣬ�ټ�BaCl2��Һ����������ɫ��������˵������SO42����2�֣�
(3)�ڳ��ȹ��ˣ�1�֣�����38��~60'����ˮϴ�ӣ�1�֣��ܵ���60'����1�֣�
(4)NaOH��Һ�������𰸾��ɣ���2�֣�
(2)2NaOH+2ClO2+H2O2��2NaClO2+2H2O+O2(2��)
SO42����1�֣� ȡ������Ӧ�����Һ���ȼ����������ᣬ�ټ�BaCl2��Һ����������ɫ��������˵������SO42����2�֣�
(3)�ڳ��ȹ��ˣ�1�֣�����38��~60'����ˮϴ�ӣ�1�֣��ܵ���60'����1�֣�
(4)NaOH��Һ�������𰸾��ɣ���2�֣�
��1����Ϊװ��I������ClO2��Ϊ�����ԭ�ϵ������ʣ���Ҫ���ÿ��������ɵ�ClO2���뵽װ�â���з�Ӧ��
��2��ClO2���������ԣ��ɽ�˫��ˮ����������������������ԭ����NaClO2������ʽΪ2NaOH+2ClO2+H2O2��2NaClO2+2H2O+O2�����ڽ���װ�â���������ж����������壬�������������������ᡣ����SO42��һ���������ữ�ļ�BaCl2��Һ��
��3������������Ϣ��֪��Ҫ��õ�NaClO2���壬�ؼ��ǿ����¶ȡ��¶�̫��̫�;���������ʡ�
��4�������ڷ�Ӧ�л����ClO2��SO2�ȴ�����Ⱦ�������Ҫβ��������
��2��ClO2���������ԣ��ɽ�˫��ˮ����������������������ԭ����NaClO2������ʽΪ2NaOH+2ClO2+H2O2��2NaClO2+2H2O+O2�����ڽ���װ�â���������ж����������壬�������������������ᡣ����SO42��һ���������ữ�ļ�BaCl2��Һ��
��3������������Ϣ��֪��Ҫ��õ�NaClO2���壬�ؼ��ǿ����¶ȡ��¶�̫��̫�;���������ʡ�
��4�������ڷ�Ӧ�л����ClO2��SO2�ȴ�����Ⱦ�������Ҫβ��������

��ϰ��ϵ�д�
 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д�
�����Ŀ