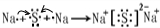��Ŀ����
��10�֣�A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ������������������A��DԪ��ͬ���壬B��CԪ��ͬ���ڣ���A��B��C��D�е�����Ԫ�ؿ��γ�ԭ�Ӹ�����Ϊ1:1�Ķ��ֻ�����ס��ҡ�������Ϊ���е����֣����ǵ�Ԫ��������±���ʾ��
|
������ |
�� |
�� |
�� |
�� |
|
���Ԫ�� |
A��B |
A��C |
A��D |
C��D |
ͨ��״���£�������Ϊ���壬�ܶ���С�ڿ�����������ΪҺ�壻�����ʺͶ�����Ϊ�����Ҷ�Ϊ���ӻ��������д���пհף�
��1�������ʵĽṹʽΪ �������ʵĵ���ʽΪ �����������������������ӵĸ�����Ϊ ��
��2������״����5.6L��������ȫȼ�������ȶ�������ʱ�ų�������Ϊ325kJ����д����ʾ������ȼ���ȵ��Ȼ�ѧ����ʽ ��
��3���о����������ʾ��������ԣ�����������ˮ�еĵ��뷽��ʽΪ ��
��10�֣��� H��C��C��H , Na+ [:H] - �� 1:2
�� C2H2(g)+ 5/2O2(g)��2CO2(g)+H2O(l)����H����1300kJ/mol
�� H2O2 H+
+HO2- ��HO2-
H+
+HO2- ��HO2- H+
+O22- (ֻҪд���˵�һ�����ɵ÷�)
H+
+O22- (ֻҪд���˵�һ�����ɵ÷�)
������������Ԫ�ء�����������ʼ�Ԫ�������ڱ��е�λ�ÿ�֪��A��B��C��D���ֶ�����Ԫ�طֱ���H��C��O��Na��
��1��������Ȳ���ṹʽΪH��C��C��H �������⻯�ƣ�����ʽΪ Na+ [:H] - �����ǹ������ƣ��������������ӵĸ�����Ϊ1:2��
��2��ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų���������5.6L��Ȳ��0.25mol������1mol��Ȳȼ�շų���������1300kJ/mol��
(3)�������ԣ����Ե��뷽��ʽ�ÿ���ţ����Ƿֲ�����ģ�����ʽΪH2O2 H+ +HO2- ��HO2-
H+ +HO2- ��HO2- H+ +O22- ��
H+ +O22- ��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�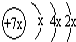 ��b��c�γɻ�����ĵ���ʽΪ
��b��c�γɻ�����ĵ���ʽΪ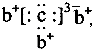 ���бȽ�����ȷ���ǣ�������
���бȽ�����ȷ���ǣ�������| A��ԭ�Ӱ뾶��a��c��d��b | B����ۺ����������c��d��a | C��ԭ��������a��d��b��c | D�����ʵ�������a��b��d��c |
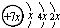 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺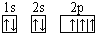

 Al��OH��3+OH-
Al��OH��3+OH-