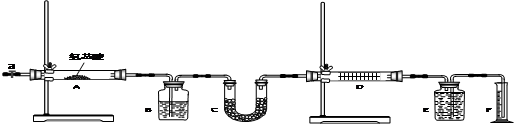
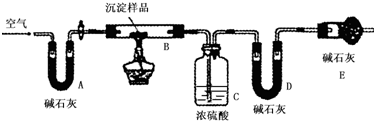
��Ŀ����
��10�֣�ʵ���Ҷ�������ij��Ʒ���������Ƶ�һ�ַ����ǣ�
����1.520g��Ʒ�м���̼�������Һ��0.13�� I2���ȷ���Һ���ڷ�Һ©������15min�����ӷ���ʽΪ��SO32����I2��2HCO3����SO42����2I����2CO2����H2O
��ȡ�������õ�ˮ��Һ������һ�������ᡢ�����ı�����ˮ��Һ����������е����ӱ������ɵ�������ӣ��õ�250mL��Һ��
���ڢ�������Һ��ȡ25mL���μӼ��ᣬ��ȥ���й�����Br2��
�ܽ���������Һ�м������Ĵ����ƣ��ټ��������ĵ⻯����Һ������Һ�����ӷ���ʽΪ��6H����IO3����5I����3I2��3H2O
���ñ��������������Һ�ζ�����������Һ��������0.1120mol/L Na2S2O3 15.10mL�����ӷ���ʽΪ��I2��252O32����2I����S4O62��
�ش��������⣺
��1��д���ڡ���������������������Ӧ�����ӷ���ʽ��
��2������ΪʲôҪ��0.13�� I2���ȷ���Һ������ֱ����I2��ˮ��Һ��
��3��������Ʒ���������Ƶ������ٷֺ�����
��1����I����3Br2��3H2O��IO3����6Br����6H����2�֣�����Br2��HCOOH��2HBr��CO2����2�֣���
��2����ֹδ��Ӧ��I2����ˮ��Һ�У�2�֣���
��3����4�֣������ϵ��SO32����2I����2IO3����6I2��12S2O32����1�֣���
n(Na2SO3)��1.409��10��4��mol����1�֣�
m(Na2SO3)��0.1775g��1�֣�
Na2SO3%��11.68����1�֣�