��Ŀ����
���ݻ�Ϊ2L���ܱ������г���2mol A�����1mol B���壬��һ�������·������·�Ӧ��2A��g��+B��g��?3C��g������2s��ﵽƽ�⣬���C�����Ũ��Ϊ0.6mol?L-1������˵������ȷ���ǣ�������
��������A��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ0.2mol?L-1?s-1
��������B��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ0.2mol?L-1?s-1
��ƽ��ʱ����A��B��ת�������
��ƽ��ʱ����B��Ũ��Ϊ0.2mol?L-1��
��������A��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ0.2mol?L-1?s-1
��������B��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ0.2mol?L-1?s-1
��ƽ��ʱ����A��B��ת�������
��ƽ��ʱ����B��Ũ��Ϊ0.2mol?L-1��
���������ݻ�Ϊ2L���ܱ������г���2mol A�����1mol B���壬����ʼʱA��Ũ��Ϊ1mol/L��B��Ũ��Ϊ0.5mol/L��
2A��g��+B��g��?3C��g��
��ʼ��mol/����1 0.5 0
ת����mol/����0.4 0.2 0.6
ƽ�⣨mol/����0.6 0.3 0.6
���v=
���㣮
2A��g��+B��g��?3C��g��
��ʼ��mol/����1 0.5 0
ת����mol/����0.4 0.2 0.6
ƽ�⣨mol/����0.6 0.3 0.6
���v=
| ��c |
| ��t |
����⣺���ݻ�Ϊ2L���ܱ������г���2mol A�����1mol B���壬����ʼʱA��Ũ��Ϊ1mol/L��B��Ũ��Ϊ0.5mol/L��
2A��g��+B��g��?3C��g��
��ʼ��mol/����1 0.5 0
ת����mol/����0.4 0.2 0.6
ƽ�⣨mol/����0.6 0.3 0.6
��v��A��=
=0.2mol/��L?s����
��v��B��=
=0.1mol/��L?s����
��ƽ��ʱ����A��ת����Ϊ
��100%=40%��B��ת����Ϊ
��100%=40%��ת������ȣ�
��ƽ��ʱ����B��Ũ��Ϊ0.3mol/L������ȷ��ֻ�Т٢ۣ�
��ѡB��
2A��g��+B��g��?3C��g��
��ʼ��mol/����1 0.5 0
ת����mol/����0.4 0.2 0.6
ƽ�⣨mol/����0.6 0.3 0.6
��v��A��=
| 0.4mol/L |
| 2s |
��v��B��=
| 0.2mol/L |
| 2s |
��ƽ��ʱ����A��ת����Ϊ
| 0.4 |
| 1 |
| 0.2 |
| 0.5 |
��ƽ��ʱ����B��Ũ��Ϊ0.3mol/L������ȷ��ֻ�Т٢ۣ�
��ѡB��
���������⿼�黯ѧƽ��ļ��㣬��Ŀ�ѶȲ���ע����������ʽ����������Ŀ��

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ
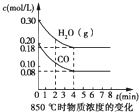 ij�о�С�����÷�ӦCO��g��+H2O��g��?CO2��g��+2H2��g����H=-41.2kJ/mol���Ʊ�CO2��H2�Ļ�����壬����һ���о�CO2��H2�Բ�ͬ�����֮�Ȼ��ʱ�ں��������µķ�Ӧ�������ش��������⣺
ij�о�С�����÷�ӦCO��g��+H2O��g��?CO2��g��+2H2��g����H=-41.2kJ/mol���Ʊ�CO2��H2�Ļ�����壬����һ���о�CO2��H2�Բ�ͬ�����֮�Ȼ��ʱ�ں��������µķ�Ӧ�������ش��������⣺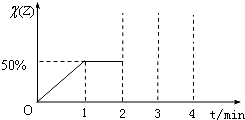

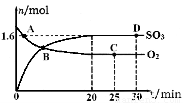
 2SO3(g) ∆H=-akJ/mol��a>0������2min�ﵽƽ��״̬����Ӧ����0.25akJ�������ж���ȷ����
2SO3(g) ∆H=-akJ/mol��a>0������2min�ﵽƽ��״̬����Ӧ����0.25akJ�������ж���ȷ����