��Ŀ����
��֪����ij�¶��£����ڿ��淴ӦX��g��+Y��g��?2Z��g�����ڸ��¶��£����ݻ�Ϊ2L�ĺ����ܱ������м���2mol X��2mol Y����1minʱ�ﵽƽ�⣬���������Z���������[����Z��]��ʱ�䣨t���仯������ͼ��ʾ����2minʱ����������м���2mol Z����3minʱ��Ӧ���´ﵽƽ����4min��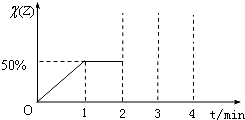
��1���÷�Ӧ��ǰ1min����X��ʾ��ƽ�����ʣ�
��2�����¶��µ�ƽ�ⳣ����
��3������ͼ�н�0��4min�ı仯����ͼ����������������ע��ͼ��������Ķ�Ӧ��ֵ��
��������1����ͼ��֪��1min����ƽ��ʱZ��Ϊ50%���ɷ���ʽ��֪����Ӧǰ�������ܵ����ʵ������䣬����ƽ��ʱZ�����ʵ�������������μӷ�ӦA�����ʵ���������v=
����v��A����
��2����������ʽ����ƽ��ʱ����ֵ����ʵ��������ڷ�Ӧǰ�������ܵ����ʵ������䣬�������ʵ�������Ũ�ȴ���ƽ�ⳣ������ʽ���㣻
��3����2minʱ����������м���2mol Z������˲��Z�������������3minʱ��Ӧ���´ﵽƽ�⣬��ЧΪѹǿ����ƽ��ʱZ�����������2minƽ������������ͬ���ݴ˻���ͼ��
| ||
| ��t |
��2����������ʽ����ƽ��ʱ����ֵ����ʵ��������ڷ�Ӧǰ�������ܵ����ʵ������䣬�������ʵ�������Ũ�ȴ���ƽ�ⳣ������ʽ���㣻
��3����2minʱ����������м���2mol Z������˲��Z�������������3minʱ��Ӧ���´ﵽƽ�⣬��ЧΪѹǿ����ƽ��ʱZ�����������2minƽ������������ͬ���ݴ˻���ͼ��
����⣺��1����ͼ��֪��1min����ƽ��ʱZ��Ϊ50%���ɷ���ʽ��֪����Ӧǰ�������ܵ����ʵ������䣬ƽ��ʱZ�����ʵ���=��2mol+2mol����50%=2mol���ʲμӷ�Ӧ��A�����ʵ���=2mol��
=1mol����v��A��=
=0.5mol/��L��min����
�𣺸÷�Ӧ��ǰ1min����X��ʾ��ƽ������Ϊ��0.5mol/��L��min����
��2��ƽ��ʱZ�����ʵ���=��2mol+2mol����50%=2mol����
X��g��+Y��g��?2Z��g��
��ʼ��mol����2 2 0
�仯��mol����1 1 2
ƽ�⣨mol����1 1 2
���ڷ�Ӧǰ�������ܵ����ʵ������䣬�������ʵ�������Ũ�ȼ���ƽ�ⳣ������ƽ�ⳣ��k=
=4��
�𣺸��¶��µ�ƽ�ⳣ��Ϊ4��
��3����2minʱ����������м���2mol Z��˲��Z���������=
��100%=66.7%����3minʱ��Ӧ���´ﵽƽ�⣬��ЧΪѹǿ����ƽ��ʱZ�����������2minƽ������������ͬΪ50%���ʲ�������ͼ��Ϊ��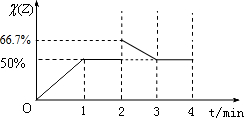 ��
��
�ʴ�Ϊ�� ��
��
| 1 |
| 2 |
| ||
| 1min |
�𣺸÷�Ӧ��ǰ1min����X��ʾ��ƽ������Ϊ��0.5mol/��L��min����
��2��ƽ��ʱZ�����ʵ���=��2mol+2mol����50%=2mol����
X��g��+Y��g��?2Z��g��
��ʼ��mol����2 2 0
�仯��mol����1 1 2
ƽ�⣨mol����1 1 2
���ڷ�Ӧǰ�������ܵ����ʵ������䣬�������ʵ�������Ũ�ȼ���ƽ�ⳣ������ƽ�ⳣ��k=
| 22 |
| 1��1 |
�𣺸��¶��µ�ƽ�ⳣ��Ϊ4��
��3����2minʱ����������м���2mol Z��˲��Z���������=
| 2mol+2mol |
| 2mol+2mol+2mol |
 ��
���ʴ�Ϊ��
 ��
�����������⿼�黯ѧ��Ӧ���ʼ��㡢ƽ�ⳣ�����㡢��ѧƽ��ͼ��Ӱ�����أ��Ѷ��еȣ�ͼ��������ѵ㡢�״��㣬ע�����õ�Чƽ��˼���������ֵĺ�����

��ϰ��ϵ�д�
�����Ŀ




 2SO3(g) ��H = ��196.6 KJ��mol-1,
2SO3(g) ��H = ��196.6 KJ��mol-1, ��ʱ��t�Ĺ�ϵ��ͼ2��ʾ�������ı�SO2 (g)��O 2 (g)��������
��ʱ��t�Ĺ�ϵ��ͼ2��ʾ�������ı�SO2 (g)��O 2 (g)��������
 < 0, ��÷�Ӧ���淴Ӧ�ܷ��Է����У� ����ܡ����ܡ������ж������� ��
< 0, ��÷�Ӧ���淴Ӧ�ܷ��Է����У� ����ܡ����ܡ������ж������� ��


 2SO3(g)
��H = ��196.6 KJ��mol- 1,
2SO3(g)
��H = ��196.6 KJ��mol- 1, ��ʱ��t�Ĺ�ϵ��ͼ2��ʾ�������ı�SO2 (g)��O 2 (g)��������
��ʱ��t�Ĺ�ϵ��ͼ2��ʾ�������ı�SO2 (g)��O 2 (g)��������